1. Introduction to the Anticancer Drug Mitomycin C and its Analogs
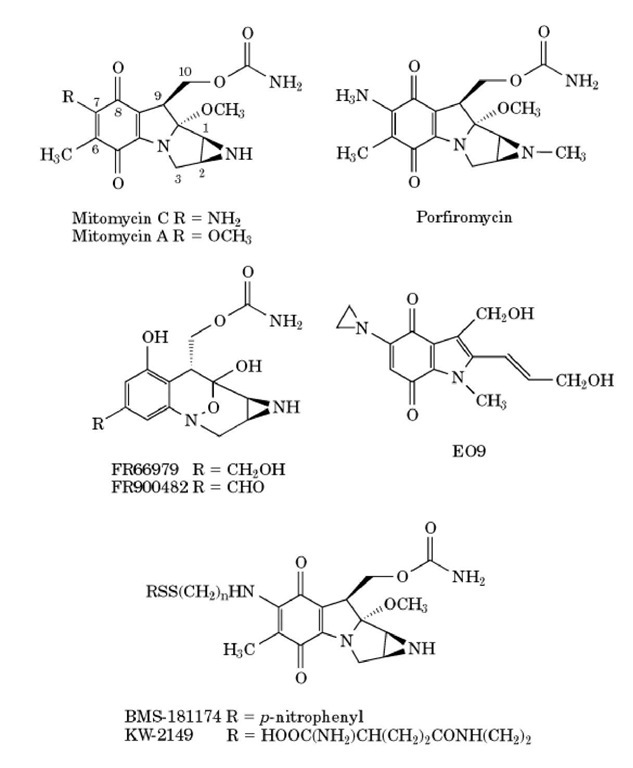
The mitomycins are a group of natural product antibiotics that were discovered in Japan in the 1950s in the fermentation cultures of Streptomyces caespitosus (1). Mitomycin C (MMC), mitomycin A, and porfiromycin represent three well-studied members of this family (Fig. 1). Shortly after the isolation of the mitomycins, their significant antitumor properties were discovered. MMC began use in clinical cancer chemotherapy in the 1960s and subsequently demonstrated a broad spectrum of antitumor activity against a variety of solid tumors (2). MMC continues to be an important component of combination chemotherapy for a variety of cancers, including carcinoma of the anal canal, non_smallcell lung cancer, esophageal and bladder cancer, and others (reviewed in (3). Bone marrow suppression is the principal dose-limiting toxicity that has limited the success of MMC as a single agent. Other members of the mitomycin family, the natural product analogs, FR66979 and FR900482, were discovered in the fermentation products of Streptomyces sandaensis no. 6897, and the semisynthetic mitomycin derivatives, E09, KW-2149, and BMS-181174 (previously designated as BMY25067) (reviewed in (4), have also been developed (Fig 1).
Figure 1. Structures of mitomycin antibotics and common analogs. Mitomycin C, mitomycin A, and porfiromycin represent three well-studied members of the mitomycin family. More recently, two other members of the mitomycin family, the natural product analogs, FR66979 and FR900482, were discovered, and the semisynthetic mitomycin derivatives, E09, KW-2149, and BMS-181174 (previously designated as BMY25067), have also been developed.
MMC has served as a model for investigating the family of mitomycins, and the vast majority of knowledge about this group of agents is derived principally from extensive laboratory and clinical studies of MMC over the past 25 years. Like most genotoxic agents, MMC binds to DNA, RNA, and other macromolecules, and MMC_DNA adducts are believed to be principally responsible for the majority of its observed biological effects. Also like many genotoxic agents, MMC requires metabolism from the parent form to a reactive intermediate to bind to macromolecules and exert its biological effects. However, unlike most other organic genotoxins, MMC requires chemical or biochemical reduction rather than oxidaton. Although the chemistry of this reduction has been well characterized in vitro, the specific biochemical pathways by which this occurs inside the cell remain controversial. MMC has been shown to induce many of the common biological hallmarks of other genotoxic cancer chemotherapy agents and genotoxic mutagens and carcinogens. MMC has been reported to inhibit DNA replication and RNA synthesis, induce mutations and the SOS response in bacteria, and induce mutations and sister chromatid exchanges in mammalian cells (reviewed in (5)).
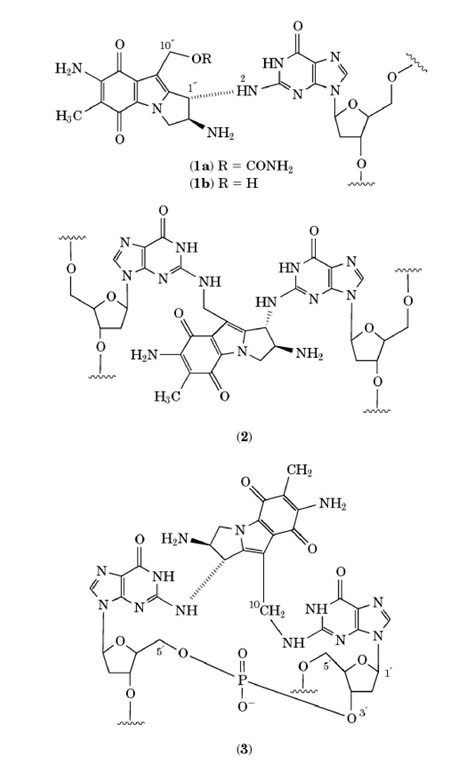
Reduced MMC covalently binds preferentially to the N position of guanine in the minor groove of duplex DNA, forming monoadducts, intrastrand crosslinks at adjacent guanines, and interstrand crosslinks at opposite guanines of adjacent basepairs, specifically in the sequence orientation of CpG, but not GpC (see Fig. 2). It is currently presumed that the CpG MMC interstrand crosslink is principally responsible for the cytotoxic and antitumor properties of MMC; however, formal evidence for this is still lacking.
Figure 2. Mitomycin C-DNA adducts. After reductive activation, mitomycin C (MMC) can form two monoadducts, i.e., a monofunctional monoadduct (1a) and a bifunctional monoadduct (1b), and also two types of cross-links, ie., an interstrand cross-link (2) at CpG sites, and an intrastrand cross-link (3) at GpG sites.
2. MMC Structure and DNA Adduct Formation
The structures of the mitomycins are unique, containing three different functional groups thought to be important for their properties and present separately in several other known carcinogens. These reactive groups, the aziridine (three membered ring attached to C and C ), carbamate (-OCONH2), and quinone (five-membered ring containing ketones at C and C ), are arranged about a pyrrolo [1,2-a]indole nucleus (see 1). In this arrangement, these functional groups are relatively inactive; however, upon chemical or biochemical reduction of the quinone by either a one- or two-electron process, a semiquinone or hydroquinone is formed, respectively, leading to the unveiling of two electrophilic sites (C1 and C10) for alkylation. According to the proposed mechanism of alkylation (reviewed in (4)), there are two pathways the MMC and follow: the "monofunctional" alkylation pathway, which results in a "monofunctional monoadduct," (1a) and the "bifunctional" alkylation pathway, which results either in a "bifunctional monoadduct" (1b) or a cross linked adduct (2 or (3). The sequence of events leading to the activation of the C1 and C10 positions of MMC involves the reduction of the quinone ring, the spontaneous elimation of methanol (driving force: formation of the pyrrole), and the opening of the aziridine ring. Once the electrophilic C1 and C10 sites are "unmasked," the next step is the highly specific nucleophilic attack by the N exocyclic amino group of guanine on the C1 position of MMC. If reoxidation of the quinone ring, by either O2 (poor redox conditions) or by the presence of excess MMC, occurs at this stage, the compound is no longer activated and is referred to as a "monofunctional monoadduct" (1a), where monoadduct refers to one addition product with DNA, and monofunctional refers to a reaction at only one functional group on the MMC ring system. This reoxidation, which is autocatalytic, occurs when the initial reduction of MMC is very slow, ie, when it can be inactivated faster than it is formed.
In the "bifunctional" pathway, after the first alkylation of DNA, the C10 position of MMC undergoes decarbamoylation that results in the activation of this position for further nucleophilic attack. If there is a second guanine present adjacent to or on the opposing strand of DNA (ie, at GpG or CpG sequences, respectively), then the N position of this guanine can serve as the incoming nucleophile on the C position of MMC, forming an N -G-MMC-N G intrastrand or interstrand crosslink (2 or 3), respectively). If there is not a second guanine in either of these two sites, H2O can serve as the incoming nucleophile and attack the C10 position, forming a "bifunctional monoadduct" (1b). The term "bifunctional monoadduct" refers to the fact that only one addition product with DNA has formed although the mitomycin complex has undergone nucleophilic reactions at two functional groups. Other less abudant MMC-DNA adducts have also been observed and their biological activity has been investigated in vitro (6).
3. Bioreduction
Due to its requirement for reduction prior to alkylating DNA, MMC is referred to as a bioreductive alkylating agent (reviewed in (7)). The bioactivation of MMC can be mimicked in vitro by chemical reducing agents such as by H2 and PtO2, which favor the formation of a monofunctional adduct with
DNA; by sodium dithionite, which favors the formation of a bifunctional adduct; or by various nicotinamide adenine dinucleotide (NAD(P)H)-dependent flavoreductases. The relative proportion of adducts vitro or in vivo depends on the sequence of the DNA and on the metabolic and redox status of the cells.
Because bioreduction is key to the cytotoxic actions of MMC, a number of laboratories have dedicated a large effort to identifying specific biochemical pathways involved in the activation of MMC. A variety of bioreductive enzymes have been implicated in the reduction of MMC, such as NAD(P)H cytochrome c reductase, NAD(P)H quinone oxidoreductase (DT-diaphorase), xanthine oxidase, xanthine dehydrogenase, and NADH cytochrome b5 reductase (reviewed in (8)). Despite this effort, much controversy still exists regarding the specific roles of each of these pathways in intact cells. The large number of divergent and sometimes contradictory in vitro and cell culture studies suggest that no single enzyme is uniquely responsible for MMC activation (reviewed in (7)). These studies also suggest that a number of variables can influence the activation of MMC, at least in vitro and in cell culture, including the cell type and cell culture growth conditions, pH, and degree of hypoxia (7, 8). For example, in vitro chemical reduction studies indicate that the pH can influence both the site of the lesions on guanine (N vs N alkylation), as well as influence the predominating bioreduction pathway (4). However, whether such parameters also influence MMC metabolism and MMC-induced biological effects in vivo is not clear, because pH, oxygen concentration, and other parameters are tightly regulated over a much narrower range under physiological conditions in the intact animal. Such studies demonstrate the limitations of in vitro and cell culture systems in modeling the complexities of a whole organism or the microenvironment of a tumor in vivo.
Several studies have suggested that DT-diaphorase and cytochrome P450 reductase are often highly overexpressed in cancer cells (7, 9). The terminology "enzyme-directed drug development" – coined with mitomycin as the paradigm – refers to an approach that seeks to identify enzymes that are overexpressed in tumors when compared with normal tissues and that can be used to preferentially target drug effects to these tumor cells (reviewed in (10)). For example, the sensitivity of bladder cancer patients to MMC has been shown to be correlated with the expression of DT-diaphorase and cytochrome P450 reductase in their bladder tumors, suggesting that the lower efficacy of intravesical MMC in these patients may be due, at least in part, to their lower expression of these enzymes (9). An important area of future study will be to determine the precise biochemical pathways by which MMC is bioreduced in tumor and normal cells, and to determine the role of each pathways in the biological effects of MMC in these target and nontarget tissues. This is critical for understanding, and hopefully being able to modulate, the efficacy of MMC and its analogs as antitumor agents against various human cancers.
4. Characterization and Measurement of MMC_DNA Adducts
The structures of the various MMC_DNA adducts have been extensively characterized by a variety of biophysical techniques—including high-performance liquid chromatography, ultraviolet absorbance, thermal melting curves, gel electrophoresis, circular dichroism, chemical footprinting, Fourier transform infrared spectroscopy, fast atom bombardment mass spectroscopy, and a variety of one and two-dimensional nuclear magnetic resonance techniques (4, 11-13)). There is general agreement from these studies that the predominant MMC_guanine monoadducts and the CpG MMC_DNA interstrand cross-link cause little or no perturbation of the DNA double helix, with the attached mitosene ring lying snugly within the minor groove of the DNA. In contrast, the GpG MMC_DNA intrastrand cross-link has recently been shown to bend the DNA helical axis by 14.6° (reviewed in (4)).
Covalent MMC_DNA adducts have been detected and isolated following in vitro reactions involving mono- or di-nucleotides, oligonucleotides, and purified DNA (eg, calf thymus DNA, Micrococcus luteus DNA, salmon sperm DNA, and poly(dG-dC)-poly(dG-dC) (11, 13)). MMC_DNA adducts have also been detected in various cell culture models, in intact animals, and in human cancer chemotherapy patients (14, 15). Due to the lack of readily available radiolabeled MMC, some studies have substituted the commercially available radiolabeled ^-methyl analog of MMC, porfiromycin. Porfiromycin has been shown to have similar reactivity and biological properties as MMC, and the corresponding porfiromycin ^-methyl analogs of the monofunctional monoadduct, bifunctional monoadduct, and cross-links of MMC have been observed (reviewed in (13)).
5. Modulation of Binding Activity at Methylated CpG Sites
Sequence context plays an important role in the types and quantity of MMC adducts formed. MMC_ DNA monoadducts and interstrand cross-links have been observed to show a strong preference for the CpG but not the 5′GpC dinucleotide sequence (reviewed in (4)). There also appears to be an enhancement of MMC cross-linking at those sequences that have a purine 5′ to the CpG site and a pyrimidine (particularly thymine) 3′ to the guanine (ie, PuCGPyr ) (4). The CpG dinucleotide sequence has some unique features that are of interest with regard to its specificity for MMC cross-linking. The CpG dinucleotide sequence is underrepresented in eukaryotic genomes compared to other G-containing dinucleotide sequences, occurring at about one fifth its expected statistical frequency (reviewed in (16)). However, clusters of CpG containing regions, referred to as "CpG islands," are often found at much higher than predicted frequencies within many gene promoters, and 60 to 90% of these sequences are methylated at the 5′ position of cytosine in vivo. Cytosine methylation is thought to be one mechanism for transcriptional regulation of genetic material. For example, the degree of cytosine methylation is often but not always inversely correlated with transcriptional activity. Cytosine methylation also alters the local structure of duplex DNA. Thus, an enhancement in MMC cross-linking at methylated CpG sites (reviewed in (4)) may be biologically significant because it has been proposed that DNA-damaging agents may heritably alter DNA methylation patterns. This would provide an epigenetic mechanism for heritable alterations in expressions of genes involved in carcinogenesis, and might also be one way in which MMC exerts its biological effects.
6. MMC-DNA Adduct Recognition and Repair
Little is known about the recognition and repair of MMC-DNA adducts in vivo. It is generally assumed that MMC-DNA adducts are repaired by nucleotide excision repair (NER), which is an important cellular mechanism that removes radiation-induced and chemically induced damage for DNA. Repair of the interstrand cross-link in particular is believed to pose a unique and difficult topological challenge to the repair machinery. However, the rapid kinetics of MMC cross-link removal from DNA in cell culture and in vivo argues for effective recognition and repair of this lesion, despite this topological problem and the relatively non distorting nature of this lesion. Several specific protein complexes from human and rodent cell nuclear extracts were found to preferentially recognize and bind to the MMC interstrand cross-link with high affinity (17). Several of these complexes appear to contain ERCC-1 (excision repair cross complementation group 1) and/or XPA (xeroderma pigmentosum complementation group A), which have both been shown to be involved in mammalian NER and to be implicated in cross-link repair (18). In vitro experiments have demonstrated that both purified XPA and the minimal DNA binding domain of XPA (XPA-MF122) were able to bind MMC-cross-linked DNA with a much greater specificity and higher affinity than to undamaged DNA (19). This occurred in the absence of other proteins from the NER complex (19). Such preferential binding may lead to enhanced recruitment of the NER machinery to the adduct site for subsequent efficient repair. Further studies of this type will be required to fully elucidate the biochemical mechanisms by which DNA interstrand cross-links formed by MMC are recognized and repaired in mammalian cells.
7. Effects of MMC on Gene Expression
In addition to its overt effects as a cytotoxic and antitumor agent, recent studies have demonstrated that lower, noncytotoxic doses of MMC can selectively modulate expression of certain target genes at doses that do not induce apoptotic or stress response, and that have no effect on expression of most other genes. For example, selective suppression of several genes associated with multidrug resistance in cancer cells was observed in several human and rodent cancer cell lines in culture (20), in a mouse tumor model in vivo (21), and in the tumors of human cancer patients in vivo (22) after a single low-dose MMC treatment. In the cell culture and rodent studies, pretreatment with MMC significantly increased the subsequent antitumor activity of a second agent such as doxorubicin or taxol (20, 21). Extensive dose-response, time course, and mechanistic studies indicated that this was principally a result of the suppression of these drug-resistance proteins by the MMC pretreatment rather than combined toxicity per se (20-22). Similar results were seen after pretreatment with other DNA cross-linking agents of divergent chemical structure, including cisplatin and carboplatin, suggesting that these effects were a result of formation of DNA cross-link adducts. These results suggest the basis for development of novel combination chemotherapy approaches, where an agent such as MMC is used as a modulator of the cancer cell phenotype in combination with other cytotoxic agents, rather than, or in addition to, its use as a cytotoxic agent (22).
In addition to cancer applications, MMC has been shown to selectively alter expression of other genes that may also be of benefit in noncancer clinical diseases. For example, MMC has been a useful model compound for examining effects of pharmacological agents on the gene regulation and protein biogenesis of cystic fibrosis transmembrane conductance regulator (CFTR), mutations of which are entirely responsible for the diseases, cystic fibrosis. At low doses, MMC induced a significant increase in both CFTR mRNA and protein expression. Because the problem in cystic fibrosis is essentially a lack of adequate CFTR functional expression, such a drug-induced increase in CFTR expression could potentially be of clinical benefit in treatment of cystic fibrosis patients (23). Identification of less toxic analogs of MMC that also upregulate CFTR expression is therefore of potential interest in this setting.
Low doses of MMC have also been shown to selectively modulate the expression of the xenobiotic-and drug-inducible genes, 5-aminolevulinate synthase (24, 25) and cytochrome P450 CYP2H1 (24, 25). and the glucocorticoid hormone-inducible phosphoenolpyruvate carboxykinase gene (24, 26)). The mechanistic basis for these selective effects on gene expression is not known. Correlative evidence indicates that these effects are primarily a result of formation of MMC-DNA adducts within or near the promoter regions of these genes. However, whether this is a result of preferential adduction of these regions by MMC (ie, higher levels of adducts at these sites), or a higher sensitivity of these particular genes to nearby MMC_DNA adducts remains to be determined.
8. Mitosene Analogs
Preclinical and clinical trials, as well as basic mechanistic studies, with the mitomycin derivatives KW-2149, BMS-181174, EO9, FR66979, and FR900482 (Fig. 1) have suggested interesting activity profiles. Due to differences in structure, these derivatives can differ from MMC in their mechanism of bioreductive activation, cross-linking efficiency, dose-limiting toxicity, antitumor activity, and/or activity against resistant cell lines (4, 27-30). The aim of such studies with MMC analogs is to develop more effective therapies, in particular treatment regimens that are active in drug-resistant cancers, that do not induce drug resistance after prolonged treatment, and that reduce nontarget tissue toxicities. However, the clinical usefulness of these newer analogs remains to be determined. For example, BMS-181174 initially showed good promise as an antitumor agent in preclinical trials, but subsequent human phase I clinical trials revealed substantial dose-limiting toxicities that precluded its continued clinical use (28). Several other MMC analogs are currently being evaluated in preclinical and early clinical trials.
9. Summary
In summary, MMC and its analog represent a unique group of compounds with respect to both their interesting chemistry and biology, and their usefulness as cancer chemotherapy agents. Although much has been learned about this class of chemicals over the past 25 years, based principally on studies of MMC, there is still much to learn about these agents, including the biochemical pathways responsible for their activation and detoxification, the specific DNA lesions induced in vivo, their repair, and their individual roles in the biological effects observed, and the further development of these agents as gene modulators and clinical cancer chemotherapy agents.