In 1957, Vallee and co-workers (1) discovered a small, cysteine-rich protein, which they termed metallothionein (MT). Metallothioneins have been found in most higher eukaryotes, fungi, and cyanobacteria. These proteins are produced in response to heavy-metal uptake, and they sequester these ions in protein-bound metal clusters. Cysteine is the sole amino acid residue that binds to metals in metallothioneins.
Regulation of metallothioneins is necessary because high metallothionein levels indiscriminately attenuate the levels of trace metals inside the cell. Metallothionein regulation is currently being studied in fungi and cyanobacteria as model metalloregulatory systems (2), where Cu or Zn function as the effector metal ions. Table 1lists the key transcription factors, all of which dissociate from their DNA metal-response elements upon metal-ion binding. The X-ray crystallographic structure (3) of the cyanobacterial metallothionein repressor, SmtB, shows a classic helix-turn-helix motif, in common with many other DNA-binding proteins. The binding of DNA by SmtB, unlike other zinc metalloregulators (see Zinc-Binding Proteins), is inhibited by the metal. Two other transcription factors, ACE1 and AMT1 (see Table 1), are both activated by Cu1+. Four copper ions congregate in an all-or-nothing manner to form a tetracopper-thiolate cluster in the N-terminus of each protein (4). The C-termini contain the transactivation domains. Cu- and S-EXAFS show that Cu-Cu distances of 2.7 A and Cu-S distances of 2.26 A characterize the clusters of both proteins and the copper-thiolate cluster in copper-loaded metallothionein. These observations point to an interesting similarity between Cu1+-bound ACE1 (AMT1) and the gene product they regulate.
Table 1. Model Transcription Factors for Metallothioneins
|
Organism |
Protein |
Metal Content |
|
SynechococcusPCC 7942 |
SmtB |
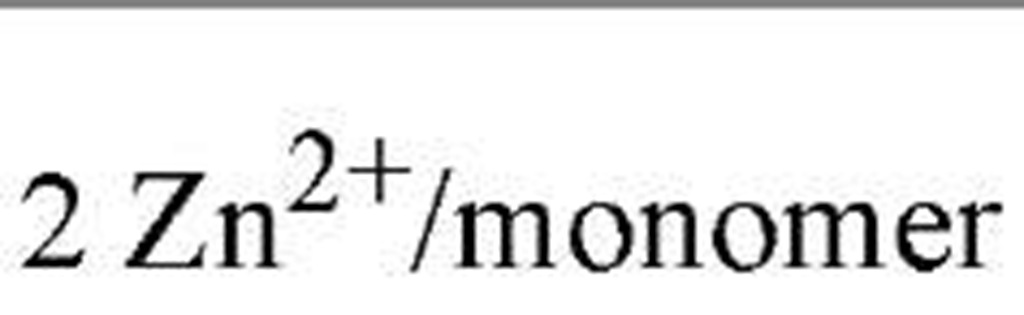 |
|
Saccharomyces cerevisiae |
ACE1 |
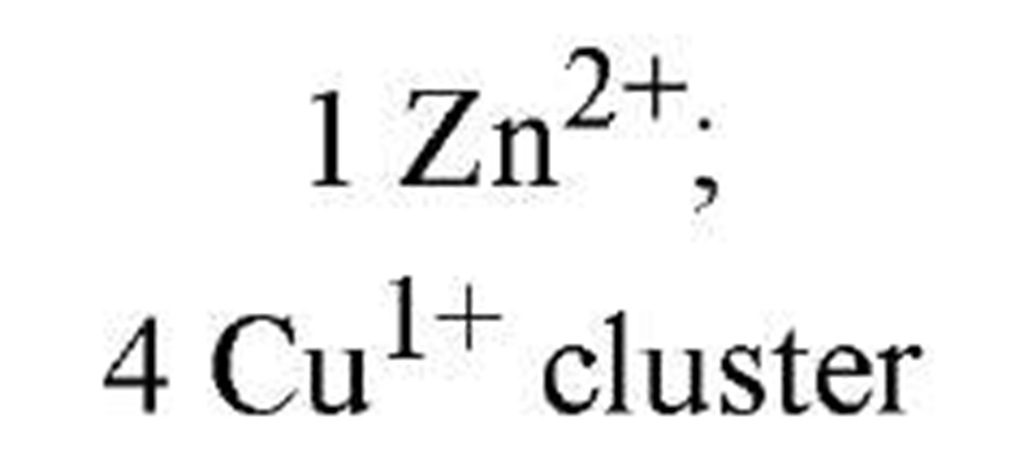 |
|
Candida glabrata |
AMT1 |
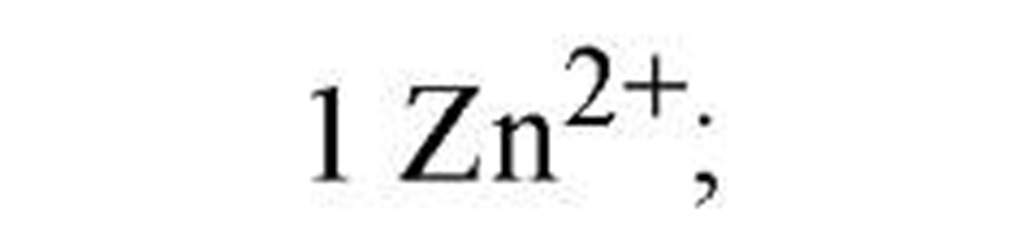 |
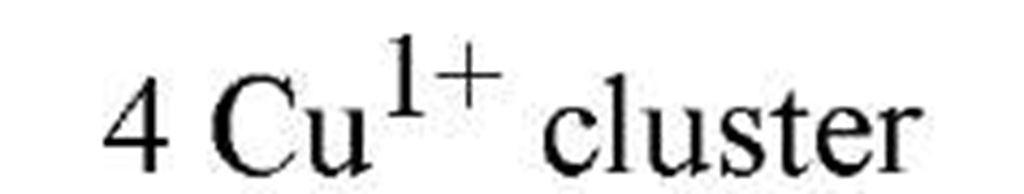 |
