The first lysozyme was discovered in 1921 by Sir Alexander Fleming, who received the Nobel Prize in 1945 for also finding the first antibiotic, penicillin. Lysozymes are widely distributed in nature, but the term "lysozyme" most usually means the protein from hen egg white. This is because it has been studied so thoroughly, in part because it is so abundant and easy to obtain in quantity. This lysozyme (EC 3.2.1.17) is the lytic enzyme that cleaves the glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine units in the polysaccharides of bacterial cell walls (Fig. 1). Consequently, lysozyme is also known as muramidase. It is a small protein (Mr = 14,307), basic (isoelectric point = 11.5), and stable (melting temperature under physiological conditions is 78°C) (see Protein Stability). It was the first enzyme containing all the 20 usual amino acids to be sequenced (1, 2). It was the first enzyme whose precise structure was determined by X-ray crystallography and whose reaction mechanism was elucidated as a result (3, 4). Thus, hen lysozyme became a model enzyme in biochemistry and biology.
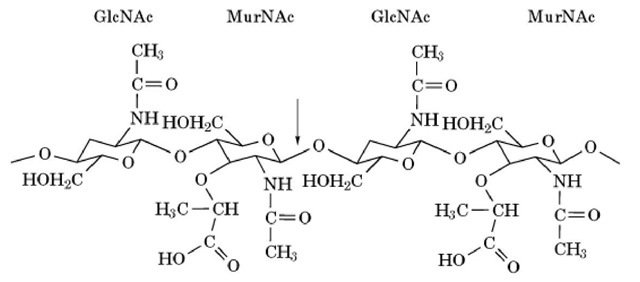
Figure 1. Structure of the bacterial cell wall, natural substrate for hen lysozyme, an alternating copolymer of N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) residues. The arrow shows the bond that is cleaved by lysozyme.
1. Structure
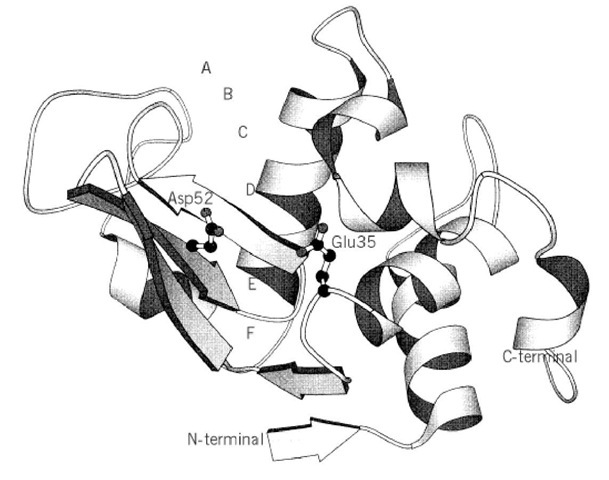
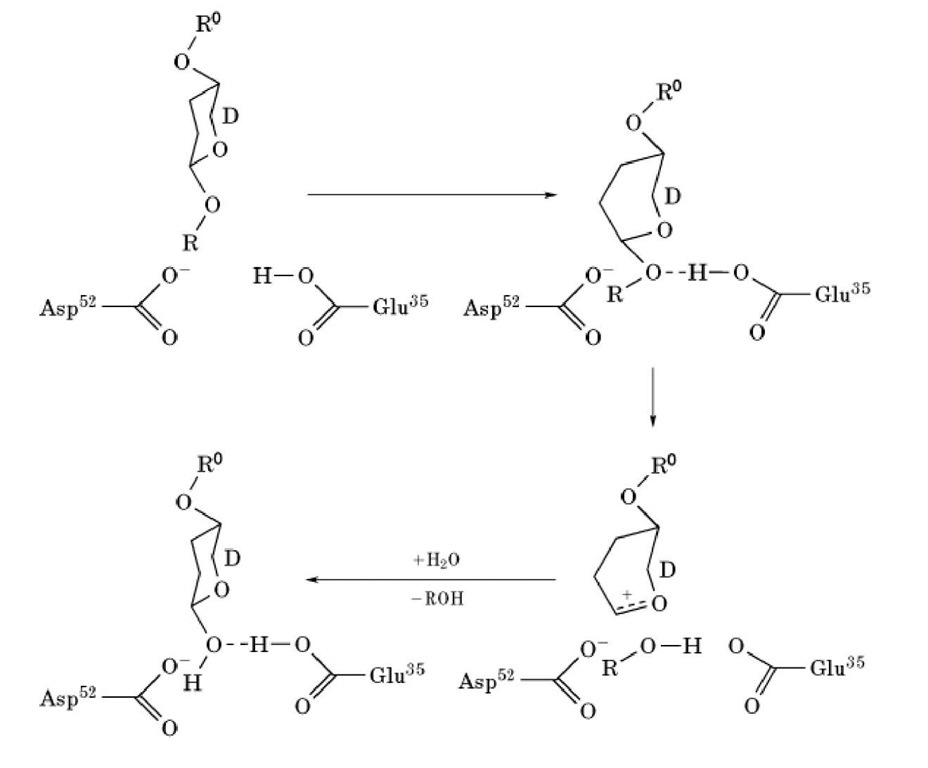
The primary structure of lysozyme was determined by Canfield (1) and Jolles et al. (2) in 1963. It consists of 129 amino acid residues, including 10 carboxyl and 7 amino groups, 11 arginine residues, 6 tryptophan residues, and 4 disulfide bonds. The three-dimensional structure of lysozyme was elucidated by Sir David Phillips’ group between 1961 and 1966 (3, 4) and is shown diagrammatically in Figure 2. The molecular dimensions of the protein are approximately 3.6 x 4.5 x 4.2mm. The structure adopts a mixed a/b fold (see Protein Structure). A deep active site cleft divides the molecule into two domains; one is almost entirely b-sheet structure (residues 4085), the other is a-helix-rich and consists of the N- and C-terminal segments (residues 1-39 and 101129). The two domains are linked by an a-helix (residues 89-99). Four disulfide bonds are formed are considered to be the primary catalytic residues. Glu35, which lies in a hydrophobic environment, participates in catalysis in its protonated form, while Asp52, which is in a hydrophilic environment, does so in its nonprotonated form. As shown in Figure 3, Glu35 donates its proton to the oxygen linking the D and E sugars. The bond between this oxygen and C1 on the D sugar is cleaved. A carbonium ion is formed on the D sugar, and this ion is stabilized by Asp52 and by the formation of an oxocarbonium ion. Formation of the planar oxocarbonium ion is favored by the distortion of the D sugar. As a result of this stabilization of the transition state, lysozyme can rapidly hydrolyze glycosidic bonds (4). This is a good example of how enzymes generally stabilize the transition state of their enzymatic reaction (see Catalysis). The reaction is completed by one-half of the cleaved product dissociating, and water replacing it and reacting with the bound intermediate. Extraneous oligosaccharides can also bind to the vacated sites and can compete with water for the last step. As a result, lysozyme has considerable transglycosylation activity.
Figure 2. The three-dimensional structure of hen lysozyme. Residues Glu35 and Asp52 are shown as the catalytic groups. The letters A-F in the active-site cleft indicate the binding subsites. The schematic figure was produced with the program MOLSCRIPT.
2. Enzymatic activity
Lysozyme hydrolyses the b-1,4-glygosidic bond between N-acetylmuramic acid and N-acetylglucosamine residues in polysaccharides (Fig. 1). Thus, lysozyme activity can be determined by the decrease in the turbidity of a suspension of dried cells of UV-inactivated Micrococcus luteus (lysodeikticus) (5). A more convenient method employing cells labeled with Remazol brilliant blue has been invented (6). Lysozyme also hydrolyzes b-1,4-glycosidic bonds between N-acetylglucosamines, and chitin derivatives can be employed as substrate. The activity is measured colorimetrically by determining the reducing sugars produced from chitin solubilized by hydroxyethylation (glycol chitin) (7). N-Acetylglucosamine (GlcNAc) oligomers ((GlcNAc)n), or their p-nitrophenylacetate derivatives, are also employed as substrates.
A reaction mechanism was deduced for lysozyme from model building the enzyme-substrate complex on the basis of the structure of Figure 2 and that of its complex with (GlcNAc^ . The active-site cleft can bind six GlcNAc residues, and the binding sites for each were designated A, B, C, D, E and F, from top to bottom (from the nonreducing end of (GlcNAc)6 to its reducing end).
Cleavage of the substrate occurs at the glycosidic bond between the residues bound to the D and E sites. The sugar ring at D site must be distorted from the normal chair conformation to a half-chair form to achieve good contacts with the enzyme. This distortion is thought to be used in the transition state of the reaction. Lysozyme residues Glu35 and Asp52 are in position near the cleavage site and between cysteine residues 6-127, 30-115, 64-80 and 76-94.
Figure 3. Reaction mechanism of lysozyme.
3. Physiological Function
Lysozyme is widely distributed in nature, so some biological functions (8) other than its primary anti-bacterial action are expected. Lysozyme is effective against bacterial and viral infections and also shows antiphlogistic activity in a number of pathological conditions. Immune-stimulating actions for lysozyme are widely found. Owing to these effects, lysozyme is employed as a medicine and as a food additive. Lysozymes are used as digestive enzymes in some organisms, such as ruminants and insects. 4. Comparative Biochemistry
Lysozymes are widely distributed in nature, from humans to viruses and plants. Almost 100 lysozymes have been sequenced. On the basis of their sequence homology, they are classified into four classes: chicken-type (c-type), viral-type (v-type), goose-type (g-type), and others. C-type lysozymes consist of 119-130 amino acid residues, and they include hen and human lysozymes. V-type lysozymes consist of approximately 140-350 residues and are found mainly in bacteriophages. G-type lysozymes are widely present in birds, and some birds have both c- and g-type lysozymes. Four g-type lysozymes have been sequenced, including those of black swan, goose, chicken, and ostrich; they have 185 amino acid residues. These three types of lysozyme are very different in size and sequence, but they are quite homologous in their three-dimensional structures.
C-type lysozymes can be subdivided into three categories: conventional, digestive, and Ca-binding lysozymes. Representative conventional lysozymes are those from hen egg white and human. Digestive lysozymes are devised to act at low pH and have low isoelectric points. Ca-binding lysozymes are interesting for their relationship to a homologous protein, a-lactalbumin, which also is a calcium-binding protein and has a three dimensional structure similar to that of c-type lysozymes. Ca-binding lysozymes include those from echidna, pigeon, and some milk. The Ca-binding loops are located at residues 82-91. These diversities in structure and function make lysozymes interesting proteins with which to analyze phylogenetic relationships.
Human lysozyme has 130 residues. Its sequence is 60% identical to that of hen lysozyme, and its properties and structure are also very similar. T4 phage lysozyme has 164 residues. It is capable of cleaving only glycosidic bonds next to N-acetylmuramic acid residues that are substituted with peptide side chains, and it also requires the presence of the N-acetamido group for activity. It is active on chloroform-treated Escherichia coli cells, and it shows no transglycosylation reaction. The catalytic residues corresponding structurally to Glu35 and Asp52 of hen lysozyme are Glu11 and Asp20, respectively, although the catalytic role of the latter has been questioned. Goose lysozyme has Glu73 as the catalytic residue corresponding to Glu35 in hen lysozyme, but apparently has no equivalent of Asp52. T4 lysozyme has been the subject of extensive protein engineering studies by Matthews et al. (9).