Studies on the involvement of lipids in signal transduction pathways and in cell regulatory mechanisms have demonstrated the regulation of key enzymes of lipid metabolism that produce important lipids and lipid-derived products that mediate signal transduction. This is best illustrated with the phosphatidylinositol (PI) cycle, where the activation of PI-specific phospholipases C results in the formation of diacylglycerol and inositol trisphosphate, both of which function as second messengers. Although this remains the best-studied example, and the anchor for the paradigm, several additional mechanisms of lipid metabolism are now appreciated to participate in significant pathways of cell regulation. Indeed, on the basis of this paradigm, all regulated enzymes of lipid metabolism appear to define novel and distinct pathways of lipid-mediated cell regulation. These enzymes include lipases, lipid kinases, and transacylases (1-4). Other entries deal with PI-specific phospholipases C and sphingomyelinases. This entry will highlight current understanding of lipases and other regulated enzymes of lipid metabolism.
1. Phospholipase D
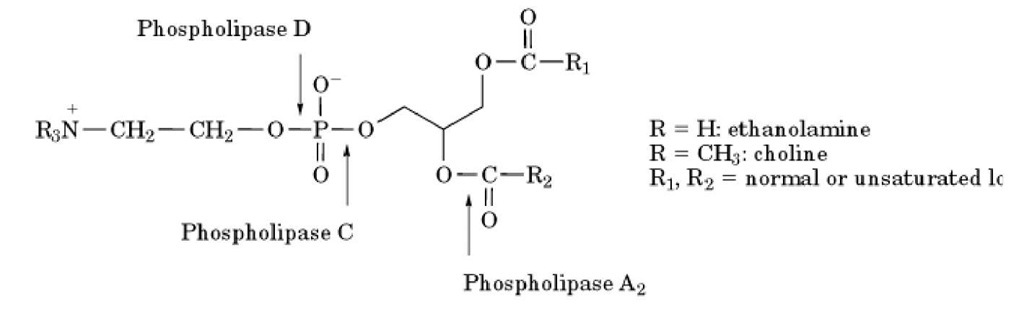
Phospholipase D catalyzes the hydrolytic cleavage of the polar head group of phospholipids (Fig. 1), resulting in the formation of phosphatidic acid and, usually, a free alcohol (such as choline when the substrate is phosphatidylcholine). Mammalian phospholipase D is activated in response to a number of growth factors and cytokines. Mechanistically, phospholipase D activation requires small G targets have been ascribed to arachidonate, including protein kinase C, phosphodiesterases, GTPases, and ion channels .
Figure 1. Sites of action of phospholipases. The structure of a phospholipid is shown with the sites of action of the main phospholipases (C, D, and A2).
Phosphatidic acid, the initial product of phospholipase D action, has been shown to exert growth-promoting activities (7). Its direct targets of action are not clearly defined, but it has been shown to regulate protein kinases, Gtpases, and other intracellular targets. Phosphatidic acid can also be further metabolized to yield lysophosphatidic acid (through a phospholipase A2 activity) or diacylglycerol (through a phosphatase activity). Lysophosphatidic acid has been shown to serve as an intercellular regulator, probably acting on a transmembrane receptor, and it has been suggested to participate in mitogenic activities (8). Diacylglycerol activates protein kinase C and participates in several signal transduction mechanisms.
In addition, phospholipase D has been implicated in vesicle formation and trafficking (6), based on the localization of its regulators (primarily the ARF family of small G proteins) in vesicles and their involvement in vesicle budding, transport, and fusion. In addition, yeast phospholipase D plays an important role in sporulation of budding yeast.
2. Phospholipase A2 and Related Enzymes of Eiconasoid Production
Phospholipase A2 attacks the acyl group at the sn-2 position of glycerophospholipids, which is usually occupied by arachidonate (Fig. 1). Several phospholipases A2 have been purified and cloned (9). These include secretory small enzymes, as well as cytosolic regulated enzymes. The best studied of the latter is cPLA2, which is regulated by calcium and by phosphorylation on serine residues. This enzyme is activated in response to a number of growth factors and cytokines and displays selectivity for arachidonate in the sn-2 position. Therefore, this enzyme has been implicated as the major regulated PLA 2 involved in signal transduction. The products of this reaction are lysophospholipid and arachidonate. Lysophospholipids may have cellular functions on their own (such as the effects of lysophosphatidic acid on mitogenesis) or may serve as precursors for platelet activating factor, which has important functions in inflammation and vascular biology. The better-studied product of the phospholipase A2 reaction, however, is arachidonate. Multiple biochemical proteins (such as ARF or RhoA), and its activation can also be effected through a protein kinase C-dependent mechanism (5-7).
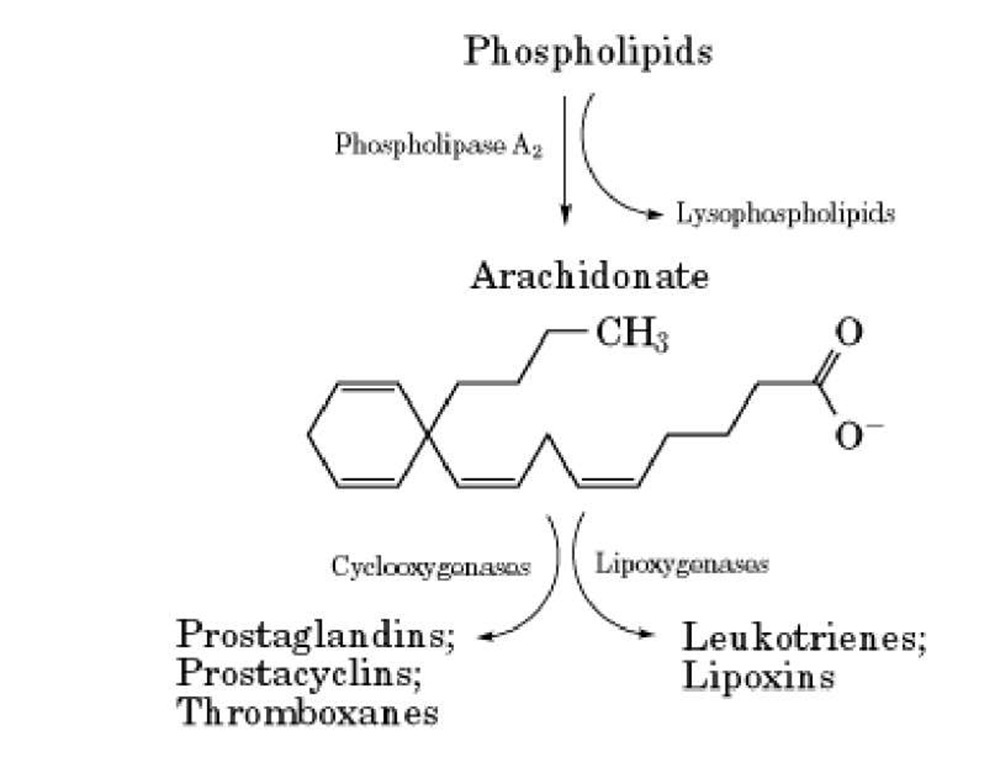
An important function for arachidonate is to serve as a precursor for the formation of eicosanoids (a collective name for products of arachidonate metabolism) (Fig. 2) (3). In particular, cyclooxygenases regulate the formation of prostaglandins, thromboxanes, and prostacyclins. These are very important intercellular mediators in platelet activation, neutrophil biology, inflammatory responses, and vascular biology. On the other hand, lipooxygenases regulate the formation of leukotrienes and lipoxins. These again serve as important intercellular messengers and regulators with important functions in inflammation, allergy, vascular biology, and blood cell activation (3).
Figure 2. The phospholipase A2 pathway and the eicosanoids. Activation of phospholipase A2 results in the liberation of from phospholipids. Arachidonate can function on its own, or it can be metabolized into two major groups of eicosanoid: either cyclooxygenases or lipoxygenases.
3. PI Kinases
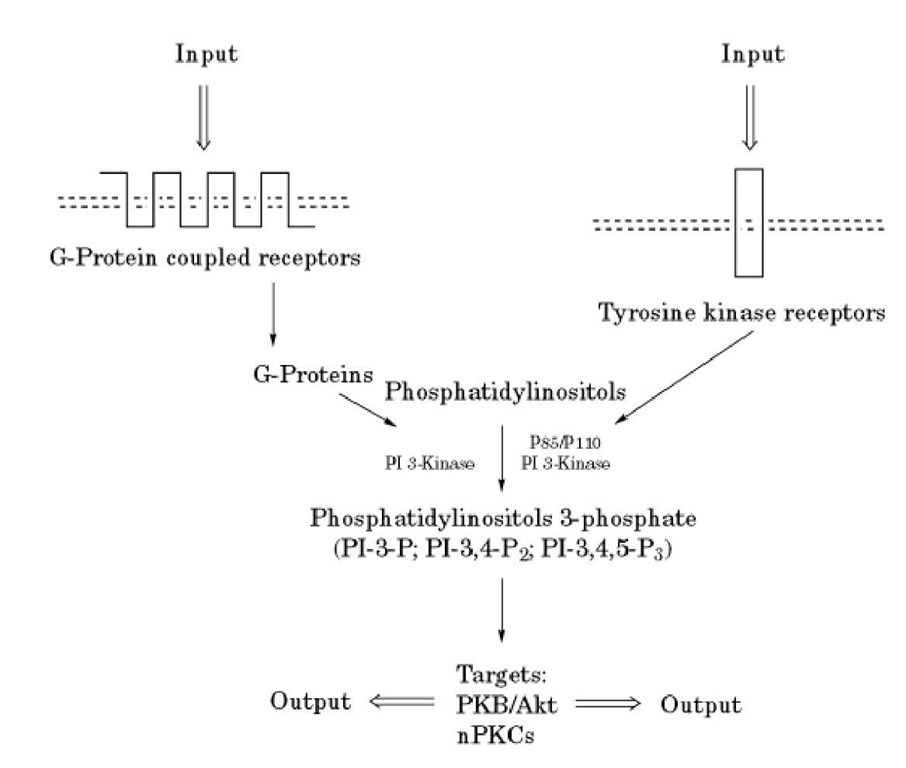
A number of kinases that phosphorylate inositol phospholipids on unique positions of the inositol ring have been described and characterized. This results in the formation of products such as phosphatidylinositol-3-phosphate, which has important roles in prevention of cell death and regulation of growth (Fig. 3). The PI-3-kinases that catalyze and regulate this reaction are activated in response to receptor stimulation through either direct tyrosine phosphorylation of regulatory subunits or through heterotrimeric G proteins (4). The phosphorylated inositol phospholipids, in turn, regulate targets involved in signal transduction, such as certain isoforms of protein kinase C and the Akt serine threonine protein kinase. PI-4-kinases, which phosphorylate inositol phospholipids at a distinct site, may play a role in vesicle trafficking.
Figure 3. Scheme of activation of PI 3-kinases. G-protein-coupled receptors or tyrosine kinase receptors activate distinct forms of PI 3-kinase, which results in the formation of 3-phosphorylated derivatives of inositol phospholipids.
4. Diacylglycerol Kinases
Diacylglycerol is a substrate for diacylglycerol kinases, whose action results in the formation of phosphatidic acid. These enzymes therefore serve to attenuate the levels of diacylglycerol and enhance the levels of phosphatidic acid, with consequences on pathways regulated by these lipid products.
5. Transacylases
An emerging group of regulated enzymes of lipid metabolism catalyze a transacylation reaction whereby acyl groups are transferred from one lipid to another. The best studied of these transacylases are the enzymes involved in the formation of platelet-activating factor, which has important functions in vascular and endothelial biology (10).