Hirudin is a protein inhibitor of thrombin in the saliva of the medicinal leech Hirudo medicinalis [see Proteinase inhibitors, protein and Serine proteinase inhibitors]. The target enzyme here seems obvious. It is a single polypeptide chain of 65 amino acid residues crosslinked by three disulfide bridges. It combines extremely tightl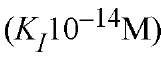 with thrombin. The crystal structure of a thrombin-hirudin complex reveals that hirudin is not a standard-mechanism canonical inhibitor. The active site of thrombin is embedded in a canyon restricting the entry of many substrates and of most trypsin inhibitors that do not inhibit thrombin. Hirudin blocks the active site, but none of its residues embeds into the Sj cavity, a hallmark of standard-mechanism canonical inhibition. The highly flexible COOH terminus of hirudin (residues 50-65) binds to the fibrinogen-binding exosite of thrombin. Many hirudin-based drugs block this fibrinogen-binding exosite rather than the active site of thrombin.
with thrombin. The crystal structure of a thrombin-hirudin complex reveals that hirudin is not a standard-mechanism canonical inhibitor. The active site of thrombin is embedded in a canyon restricting the entry of many substrates and of most trypsin inhibitors that do not inhibit thrombin. Hirudin blocks the active site, but none of its residues embeds into the Sj cavity, a hallmark of standard-mechanism canonical inhibition. The highly flexible COOH terminus of hirudin (residues 50-65) binds to the fibrinogen-binding exosite of thrombin. Many hirudin-based drugs block this fibrinogen-binding exosite rather than the active site of thrombin.
Hirudin (Molecular Biology)
Next post: His Operon (Molecular Biology)
Previous post: Hill Coefficient, Plot (Molecular Biology)
