Glutathione transferases (GSTs) are detoxication enzymes that occur abundantly in eukaryotic cells and also in prokaryotic organisms. The majority of the enzymes are found in the cytosol, but a significant proportion is also recovered in the microsomes and other subcellular membrane fractions. In mammalian liver tissue, GSTs represent several percent of the total protein content in both the cytoplasm and the endoplasmic reticulum, indicating they have important biological functions. All known soluble (mostly cytosolic) GSTs are dimeric proteins composed of two subunits of approximately 26-kDa molecular weight. In contrast, the microsomal GSTs, and probably the related membrane-bound enzymes, are trimers of 17-kDa subunits (Table 1). A plasmid-encoded GST that confers bacterial antibiotic resistance to fosfomycin is a structurally unrelated protein composed of two 16-kDa subunits. The cytosolic GSTs form both homodimers and heterodimers of closely related subunits, thereby increasing the multiplicity of GST isoenzymes .
Table 1. Structural and Catalytic Properties of Human Glutathione Transferases a
|
Hum |
in Glu |
tathion |
e Tran |
sferase |
|||||||
|
Property |
A1-1 |
A2-2 |
A4-4 |
M1-1 |
M2-2 |
M3-3 |
M4-4 |
P1-1 |
T1-1 |
T2-2 |
MGST1 (activated) |
|
Structure Subunit molecular mass |
26 |
26 |
26 |
26 |
26 |
26 |
26 |
23 |
27 |
27 |
17 |
|
(kDa) |
|||||||||||
|
Quaternary structure |
Dimer |
Dimer |
Dimer |
Dimer |
Dimer |
Dimer |
Dimer |
Dimer |
Dimer |
Dimer |
trimer |
|
Gene localization |
|||||||||||
|
Chromosome (no.) |
6 |
6 |
6 |
1 |
1 |
1 |
1 |
11 |
22 |
22 |
12 |
|
Substrate activity |
|
Aminochrome 0.04 |
0.03 |
— |
0.8 |
150 |
0.14 |
0.08 — |
|||
|
1-Chloro-2,4- |
80 |
80 |
7.5 |
180 |
220 |
7 1.4 |
105 — |
— |
24 |
|
dinitrobenzene |
|||||||||
|
Cumene |
10 |
10 |
1 |
0.6 |
0.03 3 |
7 |
3 |
||
|
hydroperoxide |
|||||||||
|
4- |
6 |
190 |
3 |
4 |
2 |
2 |
|||
|
Hydroxynon-2- |
|||||||||
|
enal |
|||||||||
|
trans- |
0.002 |
5.2 |
0.0003 0.0004 0.003 |
0.002 |
|||||
|
Stilbeneoxide |
|||||||||
a Specific activities (mol/min per mg pure protein) are given for representative substrates. Dashes represent no measurable activity; blank spaces signify missing data. Data largely compiled from the review articles by Mannervik and Widersten (1995) and Hayes and Pulford (1995).
1. Reactions Catalyzed
GSTs catalyze reactions between glutathione (GSH) and compounds with electrophilic chemical functionalities. GSH is a tripeptide, g-L-glutamyl-L-cysteinyl-glycine, that occurs in millimolar concentrations in all types of cells and in micromolar concentrations in mammalian extracellular fluids. The reactive thiol group, -SH, of the GSH cysteine residue may serve as either a nucleophile or a reductant. In both cases, the reactions are dependent on the electron-donating properties of the sulfur atom. Its reactivity is further accentuated by ionization of the thiol group to the thiolate form. One of the catalytic functions of GSTs is to decrease the pKa value of the GSH thiol group (9.2 in aqueous solution) to make GSH ionized under physiological conditions. The pKa value of enzyme- bound GSH may be lowered by several pH units. Judging from the known affinities of GSTs for GSH, it would appear that most enzymes are fully loaded with GSH in its thiolate form and ready to react with the incoming second substrate.
The common denominator for essentially all GST-catalyzed reactions is nucleophilic attack by the GSH thiolate group on an electrophilic site in the second substrate. The electrophilic center of the second molecule is usually a carbon atom, but it may also be electronegative atoms, such as oxygen, nitrogen, or sulfur. The GST-catalyzed reactions involving O, N, and S as electrophilic sites often lead to unstable products, which react with a second molecule of GSH:
where ROOH and ROH are, respectively, a hydroperoxide and the corresponding alcohol, and GSSG is the disulfide form of glutathione. In effect, such reactions accomplish reduction of organic hydroperoxides, nitrate esters, and disulfides.
The carbon-centered reactions are basically of two types: additions and substitutions. Addition reactions involve molecules such as naturally occurring a,b-unsaturated carbonyl compounds and organic isothiocyanates. The substitutions reactions involve the replacement of a chemical substituent by a glutathione group. The leaving group may be a halogenide, sulfate, or other chemical substituent with sufficient electron-withdrawing potential.
Reactions involving electrophilic carbon centers usually lead to GSH conjugates that may be excreted from the cell via ATP-dependent membrane-associated transporters, such as the multidrug resistance-associated protein (MRP) (see Drug Resistance), for subsequent disposition through the gut or the urinary tract. For urinary excretion, GSH conjugates are metabolically transformed by cleavage of the two peptide bonds of the glutathione moiety, followed by acetylation into the corresponding ^-acetyl-cysteine derivatives, mercapturic acids. These reactions are accomplished through the action of g-glutamyl transpeptidase, a dipeptidase, and an acetyltransferase (see Glutathione).
2. Discovery
GSTs were discovered in mammalian liver as enzymes catalyzing aromatic nucleophilic substitution reactions in which GSH replaces a halogen atom in an aromatic compound, such as a chloronitrobenzene derivative (1, 2). When other classes of organic compounds, with epoxide, alkene, and other electrophilic centers, were investigated, additional enzyme-catalyzed GSH conjugations were identified (3). The introduction of improved separation techniques lead to the identification of multiple forms of GST catalyzing the same chemical reaction, i.e. isoenzymes in the original sense of the term (4, 5). Subsequent work with purified enzymes demonstrated that although each enzyme form has its characteristic substrate specificity profile, most GSTs catalyze reactions with a wide range of electrophiles, and the majority of the active compounds are at least to some degree substrates for several GSTs.
3. Substrates
GSTs as a family of enzymes are capable of catalyzing reactions involving literally thousands of electrophilic compounds, including many of xenobiotic origin. In this respect, the GSTs display similarities to antibodies, which may evolve to interact with and provide protection against an almost unlimited number of molecular structures.
The majority of the known GSTs are regarded as detoxication enzymes and are catalytically active with a large number of xenobiotics, including epoxides of potent carcinogens, such as benzo[a] pyrene and aflatoxin. Naturally occurring substrates include a wide variety of genotoxic compounds, such as epoxides, activated alkenes, hydroperoxides, and quinones, all products of oxidative metabolism. Organic isothiocyanates are abundant in edible plants, from which they are released in high concentrations by injuries caused by insects and microbial infections.
4. Purification
Affinity chromatography methods have been developed for the purification of GSTs. Most purification procedures involve affinity matrices based on glutathione derivatives as ligands. Immobilized £-hexylglutathione, first used for the purification of glyoxalase I, is linked via the a-amino group of the g-glutamyl residue of glutathione to a suitable matrix (6). This adsorbent potentially combines the affinities of the H subsite of the GST active site (see below) for the hydrophobic hexyl substituent and of the G subsite for the GSH moiety. Another commonly used affinity matrix makes use of GSH immobilized via its thiol group (7). Elution of bound GSTs may be effected by competition using GSH derivatives with affinity for the active site of the enzyme. Alternative elution procedures involve changing the pH of the eluent to extreme values, such as pH 10 or pH 2. When applied to crude tissue fractions, affinity chromatography based on these matrices will yield mixtures of GSTs, which can be resolved by other methods. For separation of the dimeric proteins in a functional form, chromatofocusing or ion-exchange chromatography may be used. For analytical purposes, the subunits may be resolved by reversed-phase high-performance liquid chromatography (HPLC).
Less specific affinity methods used for the purification of GSTs include Orange A dye chromatography and metal chelate chromatography. The latter methods have proved useful for the isolation of the several GSTs that do not bind to affinity matrices based on glutathione derivatives.
5. Nomenclature
The International Enzyme Commission has recommended the name RX:glutathione R-transferase (E.C. 2.5.1.18) or the trivial form glutathione transferase, which has been adopted here. Another designation commonly used is glutathione ^-transferase, but this is inconsistent with the rational name, since the group transferred is not the sulfur of glutathione. The generally accepted abbreviation GST has probably contributed to the persistence of the S-prefix.
In the time period when the first GSTs were discovered, the enzymes were named in accord with the reactions used for their identification: glutathione S-aryltransferase, glutathione S-alkyltransferase, and so on. With the exception of the membrane-bound transferase catalyzing the glutathione conjugation of leukotriene A4, named leukotriene C4 synthase, the principle of designating GSTs by their substrates or products has been abandoned, because most enzymes have very broad substrate specificities so that accurate distinctions cannot be made.
Another nomenclature system arose from the finding that the cytosolic "Y-fraction" of rodent liver could be separated by SDS-PAGE into components that corresponded to GST subunits. These subunits were distinguished by lower indices, namely Ya, Yb and so on, and were further subdivided into Yal, Ya2. Subunits with the same mobility but different structures were identified.
A rational nomenclature for the cytosolic or soluble GSTs is based on two principles. First, the native enzymes occur as binary combinations of GST protein subunits, so that the functional properties of the dimeric protein reflect those of its constituents subunits (8). Second, the GSTs can be divided into different classes primarily based on similarities in their amino acid sequences (9). In mammals, seven classes of soluble GSTs have been identified thus far: Alpha, Mu, Pi, Theta, Sigma, Kappa, and Zeta. Sequence identities within a class are normally >50%, but they may exceed 95%. Classification of GSTs from other biological species requires further structural studies, but designations for insect enzymes (Delta) and bacteria (Beta) have been used.
In the current nomenclature (Table 1), the soluble mammalian GSTs are denoted by a capital Roman letter indicating the class (A, M, P, T, S, K, and Z) as well as two hyphenated Arabic numerals showing their subunit composition (10). Thus, GST A4-4 is a homodimer of subunit 4 within the Alpha class, and GST A1-2 is a heterodimer composed of subunits 1 and 2 from the same class. There may also be reason to distinguish allelic variants: Human GST M1a-1b is a heterodimeric member of the Mu class composed of variants of subunit 1 in the Mu class encoded by the GSTM*A and GSTM*B alleles. A prefix may be used to identify the biological species from which the enzyme derives, such as "h" for the major human enzyme hGST A1-1.
The mammalian membrane-bound GST first isolated from the microsome fraction is a trimeric enzyme composed of identical subunits and cannot readily be accommodated within the nomenclature system used for the soluble GSTs. It is simply referred to as microsomal GST (MGST1). The membrane-bound GST specifically catalyzing the conjugation of leukotriene A 4 is referred to as leukotriene C4 synthase.
6. Structure
Three distinct categories of GST protein structures are known.
6.1. Soluble GSTs
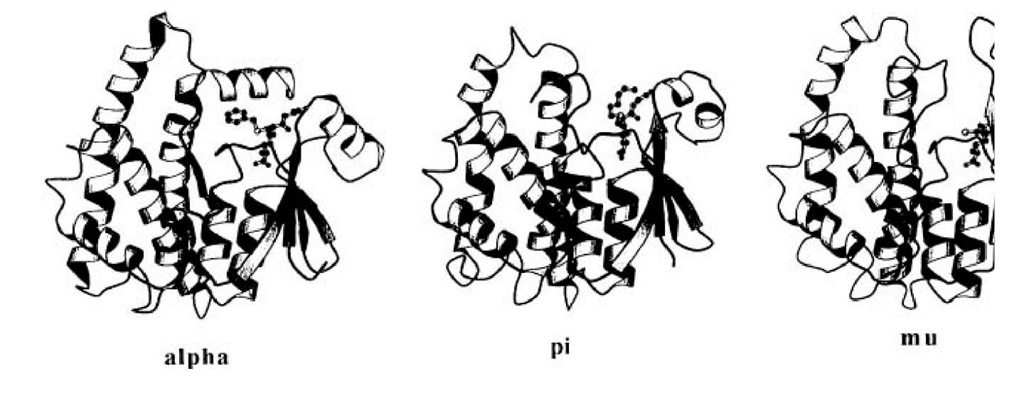
The first category includes the soluble GSTs, which are generally dimers of equal-sized subunits, each containing an active site composed of a pocket for glutathione (the G site) and a pocket for the largely hydrophobic electrophilic substrate (the H site) (11). Each subunit consists of two structural domains; (a) an ^-terminal domain including the first one-third of the primary structure and (b) a domain formed essentially by the remaining two-thirds of the amino acid sequence (Fig. 1). The first domain is folded into a mixed b-sheet flanked by a-helices and provides the structural basis for the G site. The second one is formed by helical segments and provides the major contributions to the H site. The fold of the ^-terminal domain is essentially the same as the fold of thioltransferase (glutaredoxin) and selenium-dependent glutathione peroxidase. Another salient feature of the dimer is a deep cleft between the subunits that may serve as a binding site in addition to the active site cavities. The fully formed dimer appears to be a structural requirement for a catalytically active GST.
Figure 1. Schematic drawings of alpha (GST A1-1), pi (GST P1-1) and mu (GST 3-3) structures with their ligands. The been chosen to show the 2 domains and C-terminal region.
All known structures contain an ^-capping box sequence, (Ser/Thr)-X-X-Asp, as well as a hydrophobic staple motif formed by flanking amino acid residues in the core of the folded structure. As evidenced by mutational analyses of protein folding (12), these structural signatures appear to play an important role in the nucleation and orientation of a centrally located a-helix.
6.2. Manganese-Dependent Plasmid-Encoded GST
The second category is represented by the manganese-dependent plasmid-encoded GST that is active with the antibiotic fosfomycin (13). It is a homodimer structurally related to mammalian glyoxalase I, a GSH-linked zinc protein whose structure has been determined (14). This GST is structurally unrelated to other known GSTs.
6.3. Membrane-Bound GSTs
The third category contains the microsomal MGST1 and, presumably, the additional membrane-bound GSTs. The detailed structure of MGST1 is unknown, but clearly it is not similar in structure to the soluble GSTs. The protein is a homotrimer, and the identical monomers appear to have a high content of a-helical structure.
7. Catalytic Mechanism
GSTs bind the peptide moiety of GSH in a well-defined manner so that they are specific for this thiol substrate. For all GSTs investigated, the a-carboxylate group of the g-glutamyl residue of GSH is required for the thiol group to serve as a substrate; when bound in the G subsite, the carboxylate appears to have a negative charge that is not balanced by a positively charged protein group. These observations suggest a contribution of the substrate carboxylate group to catalysis. At least for some GSTs, there is also evidence that binding of GSH induces a conformational change in the protein that promotes binding of the second substrate.
Binding of GSH to the G subsite affects the ionization of its sulfhydryl group, thereby making it more reactive. In addition, interactions with a hydrogen-bond donor in the active site may stabilize and orient the thiolate group for optimal interaction with the electrophilic substrate. In this manner the enzyme promotes catalysis by selecting a suitable ground-state conformation of GSH.
Binding to the H subsite also results in desolvation of the electrophilic substrate and selection of an orientation favorable for reaction with the sulfur of GSH. Some GSTs have tyrosine or other residues that afford binding specificity, as well as activation of substrates by hydrogen bonding and other electrostatic interactions.
In some, but not all, GST-catalyzed reactions, stabilization of the transition state contributes to the catalytic efficiency. Thus, the common mechanistic feature of GSTs is the activation of GSH, whereas other components of the catalytic process may differ from substrate to substrate and from enzyme to enzyme.
8. Binding Function
A ligand-binding function of GSTs of possible physiological significance was originally found in the protein fraction Y, of rat liver cytosol, which binds organic compounds such as certain carcinogens, dyes, and corticosteroids. The abundance of the protein (several percent of the total cytosolic protein) and its broad specificity for binding of a variety of ligands suggested a function similar to that of serum albumin in blood plasma and led to the name of "ligandin" (intracellular albumin). In the current nomenclature, ligandin corresponds to the Alpha class GST A1-1, with some contribution of the heterodimer GST A1-2. Corticosteroid binding has also been associated with GST isoenzymes of the Mu class.
The binding function of GSTs has often been ascribed to the H subsite, at which the enzyme binds the various electrophilic substrates. Some nonsubstrate compounds, especially small molecules, can bind at the H subsite with a stoichiometry of one molecule per subunit. However, X-ray crystallography studies have also shown that the deep cleft between the two GST subunits serves as an additional binding site of the dimeric structure, explaining the stoichiometry of one ligand per two subunits observed for certain ligands.
As binding proteins, GSTs have been suggested to facilitate the intracellular transport of endogenous molecules, such as heme and bilirubin, as well as other compounds of low solubility in an aqueous environment. The transport through cellular membranes of dyes, such as indocyanine green and bromosulfophthalein, which have been used in clinical liver function tests, may also be facilitated by GSTs. An auxiliary role in active-transport processes involving MRP and other membrane transport systems may also be suggested. However, the full significance of GSTs as binding proteins needs to be further clarified.
9. Molecular Evolution
The presence of GSH is a signature of aerobic organisms (15), as is the occurrence of GSTs. Elucidation of the details of the phylogeny of GSTs requires additional structural information. The soluble (cytosolic) GSTs are found ubiquitously, however, and all their three-dimensional structures determined, from bacteria to higher plants and mammals, have the same two-domain fold, even though sequence similarities may be undetectable. A common ancestry followed by divergent evolution is therefore indicated. The most recently discovered Kappa, Theta, and Zeta classes of
GST appear to be evolutionary precursors of the more abundant mammalian enzymes from the Alpha, Mu, and Pi classes. It has been proposed, in accordance with the symbiont theory of organelle evolution, that the Kappa class GST found in mitochondria may be a descendant from a bacterial GST. The structural similarities between the ^-terminal domain of the soluble GSTs and other GSH-linked proteins (see text above) lends support to the hypothesis that a GSH-binding protein is a functional unit that has been combined with other functional protein units and evolved to serve diverse biological functions. The GST structure itself has evolved to fulfill alternative functions, such as that of crystallins in the eye lens of cephalopods. The various GST isoenzymes may have arisen by gene duplications, recombination of DNA segments, and random mutations .
The membrane-bound GSTs and the plasmid-encoded Mn-dependent GST seem to have undergone convergent evolution to acquire the property of catalyzing the nucleophilic attack of GSH on electrophiles. Thus, three evolutionarily unrelated superfamilies of GSTs have been recognized.
10. Differential Tissue Distribution
GSTs are found in all tissues investigated in multicellular organisms, but the expression of the multiple genes differs from tissue to tissue. Also, the enzyme distribution changes with time during the development of embryonic to adult organs. Some GSTs have a reasonably general tissue distribution, whereas others have a more restricted occurrence. Also intracellular spatiotemporal differences in GST distribution have been noted. The soluble GSTs are found mainly in the cytoplasm, but may also occur sometimes in the nucleus. The mammalian Kappa class GST is found in the mitochondrial matrix.
The differential distribution of GSTs may have toxicological consequences, since the amount and nature of a given GST will determine the capacity of a cell to resist insults from genotoxic and carcinogenic electrophiles. The presence or absence of a particular enzyme will influence the resistance phenotype of a tissue. In cancer cells, GSTs may contribute to drug resistance, particularly against alkylating cytostatic agents.
11. GST Genes
Mammalian cells generally appear to have more than 20 GST genes, each encoding a distinct GST subunit. In humans, as well as in rodents, the genes have been shown to be distributed on different chromosomes, and the loci for related GSTs from the same class are generally clustered on the same chromosome. In addition, pseudogenes may occur close to the active genes. This distribution supports the classification adopted for the GSTs and is in agreement with the proposal that they have diversified in evolution by gene duplication and DNA recombination.
Genetic polymorphisms are known, and the human genes for GST M1-1 and GST T1-1 are deleted in approximately 50% and 20%, respectively, of the population. The resulting enzyme deficiencies lead to increased sensitivities to gene modifications and chromosome aberrations by certain chemical agents, and they probably also lead to increased risk of contracting certain forms of cancer. On the other hand, the activity of GST T1-1 promotes the formation of mutagens from ethylene dihalogenides, and the absence of the enzyme may be advantageous under some circumstances.
12. Regulation of Gene Expression and Induction of GST Activity
The differential expression of the GSTs is governed by hormones and other factors that regulate transcription of the genes. A range of enhancers and other regulatory elements, shown to mediate responses in other genes, have also been shown to be functional in GST gene regulation. Particular attention has been given to the antioxidant response element, ARE (also called the electrophile response element), which has proven effective in the induction of the activity of several detoxication enzymes in mammalian systems. An extremely broad range of chemical compounds containing an electrophilic group appear to serve as inducers via ARE, suggesting that enzyme induction is a natural response to exposure to electrophiles.
13. Biological functions
GSTs serve in cellular detoxication systems and constitute the major line of defense against chemical electrophiles, many of which are genotoxic and carcinogenic. The substrates include numerous xenobiotics or their metabolically activated products—for example, epoxides of carcinogenic polyaromatic hydrocarbons. Plants produce a variety of toxic compounds as a defense against attack by insects and microorganisms, and some of them can be inactivated by GSH conjugation. For example, many edible plants produce toxic organic isothiocyanates that are substrates for human GSTs. Like their mammalian counterparts, plant GSTs are inducible enzymes that serve protective functions, one of which is to provide cellular resistance to electrophilic herbicides.
Among the biologically most important substrates are numerous oxidation products of normal cell constituents, such as lipids, nucleic acids, catechols, and other aromatic or unsaturated chemical compounds. Free-radical reactions, accompanied by chemical transformations involving reactive oxygen species, lead to a variety of electrophilic products that may cause damage to DNA and proteins. Lipid peroxidation gives rise to aldehydes and activated alkenes, and cellular oxidation of catecholamines produces ortho-quinones. These naturally occurring oxidation products, considered to be etiological factors in the development of atherosclerosis, cataract, cancer, Parkinson’s, and other degenerative diseases, are all substrates of GSTs. Human GST A4-4 has particularly high activity with the lipid peroxidation product 4-hydroxynon-2-enal (Table 1), and human GST M2-2 is the most efficient enzyme with the ortho-quinone aminochrome derived from dopamine.
In addition to their catalytic functions, GSTs may serve as binding proteins (ligandin) that facilitate cellular transport of organic molecules. Some of the most abundant enzymes, such as GST A1-1, appear particularly well-suited for this purpose.
14. Biotechnology Applications
The soluble GSTs are generally stable proteins that can be produced in large quantities by heterologous expression in Escherichia coli. Thus, the enzymes have potential for development into useful recombinant proteins of value for biotechnical, agricultural, and medical applications. Both the catalytic and binding properties can be explored. Catalysts and binding proteins with novel specificities can be designed by a combination of mutagenesis and selection methods.
15. Glutathione Transferase as a Fusion Protein for Expression of Other Proteins
In the expression of proteins by recombinant-DNA methods, it is frequently found that the protein product is obtained in poor yield owing to low solubility, improper codon usage, proteolytic degradation, and so on. In many cases, these problems may be overcome by producing the desired protein product in fusion with a GST molecule. DNA encoding GST with a cleavable C-terminal linker to the protein to be expressed is ligated to the target DNA. GSTs are normally stable and soluble proteins that can be expressed at high levels in many cell types, and the recombinant fusion proteins usually retain these properties. The fusion protein can be purified by affinity chromatography on immobilized glutathione derivatives and, after liberation of the desired protein by proteolytic cleavage, the GST moiety can be removed by a second affinity chromatography. By this two-step purification, the desired protein may be obtained in pure form.