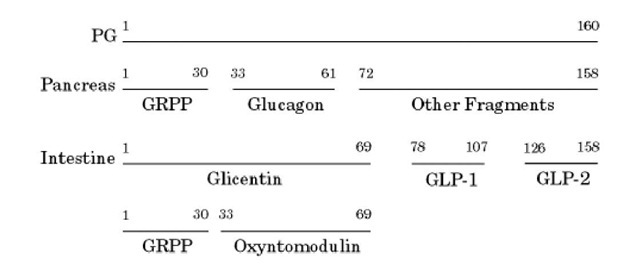
Glucagon is a 29-residue, single-chain peptide hormone (MW 3485) (1). Its gene is located on human chromosome 2, and its primary structure is given in Figure 1. Glucagon has a regulatory function in glucose metabolism; in general, its effects are opposite those of insulin. It is synthesized by a-cells in the islets of Langerhans of the pancreas and in L-cells of the intestinal mucosa as a very much larger pre-pro-glucagon molecule of 180 amino acid residues. The signal peptide is removed proteolytically upon translocation of the nascent polypeptide chain into the endoplasmic reticulum, to generate proglucagon (160 residues); this contains within it other peptides. It is processed by the pro-hormone convertases 1/3 (PC-1/3) and PC-2 (see Peptide Hormones) differently in the two tissues (2). In the pancreas, proglucagon (PG) yields glucagon and glicentin-related peptide (GRPP). In the intestinal mucosa, glucagon-like peptides-1 (GLP-1) and -2 (GLP-2) and glicentin (a 69-residue peptide containing glucagon and GRPP) are produced (Fig. 2).
Figure 1. The primary structure of glucagon.
Figure 2. Proteolytic processing of proglucagon (PG) in the pancreas and in the intestine. GRPP: glicentin-related peptide. GLP: glucagon-like peptide.
The secretory granules of the a-cells contain glucagon and discharge it by exocytosis. Active GLP-1, a potent hormone that also antagonizes insulin action, is secreted from the intestine (3-6). Consequently, differential processing of pro-glucagon gives rise to two different active peptides in the regulatory pathways. Both glucagon and GLP-1 have their own specific receptor; their genes have been cloned and the proteins characterized in structure-function and signal transduction studies (7, 8).
In general, glucagon and GLP-1 antagonize many actions of insulin and regulate the levels of blood glucose. Like insulin, glucagon lacks a plasma carrier, and its circulation half-life is short, approximately 3 to 6 minutes. Glucagon is removed from the circulatory system primarily by the liver and kidney. The predominant effect of glucagon is on the liver, because it is the first organ perfused by blood with the pancreatic secretions. Glucagon binds to receptors on the plasma membrane and couples through heterotrimeric G-proteins to GTP-binding proteins and adenylate cyclase. The resultant increases in cyclic AMP (cAMP) and cAMP-dependent protein kinase A (PKA) levels reverse the effects of insulin on the liver. These increases elevate the circulating glucose levels by stimulating the output of glucose from the liver by glycogenesis and promoting glycogenolysis. Glucagon secretion is inhibited by glucose. In contrast to insulin, which promotes energy storage, glucagon releases energy. It also has some effect on lipid metabolism, by reducing fatty acid synthesis and increasing fatty acid oxidation and ketogenesis. Glucagon secretion does not markedly fluctuate throughout the day, but the ratio of insulin to glucagon mediates phosphorylation or dephosphorylation of enzymes that regulate metabolism.
In type I diabetes, the stimulating effect of hypoglycemia on glucagon secretion is reduced. In these diabetic patients, glucose fails to inhibit glucagon secretion, but insulin injections restore the effect. On the other hand, somatostatin is an inhibitor of both glucagon and insulin secretion. A potent glucagon receptor antagonist may have therapeutic use in hypoglycemia. Consequently, the roles of glucagon and GLP-1 in glucose metabolism are presently an area of diabetes research.