The fungi represent one of the most diverse groups of organisms encountered in the natural world; approximately 70,000 different species have been described although estimates as high as 1.5 million fungal species are likely to exist. Even though a large fraction of fungi are believed to be unculturable, their importance to the natural environment of the planet cannot be underestimated. Thus, a clear understanding of their physiology at a molecular level seems prudent if not essential. The past 10 years have seen an explosion of information in this field, due primarily to the ability to transform a wide variety of these previously intractable organisms. Yet, even with the exponential advances in recombinant techniques (especially sequencing technologies) gained over the past several years, filamentous fungi appear to be the forgotten model system; perhaps it is because the completion of the first eukaryotic genome, Saccharomyces cerevisiae, was a fungus, albeit a yeast, or perhaps it is because of the success of the human genome project that the perception is that we need not look at additional fungi as model organisms. However, while Saccharomyces provides an excellent framework for studying eukaryotic systems, it clearly lacks some critical parameters that filamentous fungi can elucidate, in particular, multicellular differentiation and pathogenicity. In addition, it should be kept in mind that a large percentage of the expressed sequence tags (ESTs) identified from filamentous fungi do not have detectable homology to sequences already present in any of the available databases, including the Saccharomcyes genome). Therefore, the filamentous fungi as a group provide a complex and somewhat unique perspective of the microbial world that should not be overlooked.
Two of the most well known filamentous fungi, Aspergillus nidulans and Neurospora crassa, are exceptional model organisms. They have a long genetic history and are continuing to be pursued as excellent subjects in which to study development, gene regulation, circadian rhythms, and genome structure. As the functions and interactions of proteins and intracellular molecules is elucidated from a variety of organisms, including the fungi, humans appear to have more in common with their distant eukaryotic ancestors than previously thought. It is important to keep in mind that the filamentous fungi are part of the group of organisms that include the yeasts, Saccharomyces cerevisiae and Schizosaccharomycespombe, two of the best understood eukaryotic organisms from a genetic and molecular standpoint. In general, the molecular biology of the entire kingdom may be viewed as more similar than dissimilar; not only are there are two-component signal transduction systems, transcriptional inducers and repressors, but the conservation of protein sequence is remarkable in many areas. Wherever commonalities have been searched for they have been found, with a few exceptions that may yet prove to be due to insufficient data.
1. Why Study Fungi?
Fungi are a biochemically diverse set of organisms, producing a wide variety of acids and degradative enzymes that support their absorptive lifestyle and play an essential role in the degradative processes of our entire ecosystem. They decompose numerous substrates by secreting a wide variety of very efficient degradative enzymes, thus providing an efficient means to recycle much of the earth’s biomass. In addition, their fermentative capabilities provide a means to bake bread and ferment a wide variety of fruits and vegetables. The fungi have also been exploited commercially as efficient producers of enzymes such as glucoamylase, lipase, cellulase, and pectinase. In addition, fungal secondary products have been used in the synthesis of numerous pharmaceuticals, including penicillin, cyclosporin, and mevalonin.
On the other hand, some fungi can have a devastating effect: their uncontrolled growth can irrevocably damage entire forests, crops, or agricultural feed products. Some of the most intensely studied fungi are phytopathogenic; Magnaporthe grisea (pathogen of rice), Ustilago maydis (corn), Cryphonectria parasitica (chestnut blight), etc. Aflatoxin, a single secondary metabolite produced by Aspergillusflavus, causes millions of dollar of damage to the corn industry annually. In terms of human disease, medically important species of fungi are increasing in prevalence due to a dramatic rise in the incidence of immunocompromised patients. The most significant human pathogen is C. albicans, a dimorphic organism that switches between a yeast-like growth phase not unlike that of its distant cousin, S. cerevisiae, to a hyphal growth phase that has been associated with its virulence in vivo. Other well-known fungal pathogens include Aspergillus fumigatus, Blastomyces dermatitidis, Coccidioides immitis, Cryptococcus neoformans, Histoplasma capsulatum, and Pneumocystis carinii. Many are associated with high mortality rates. All of these fungi are either filamentous or dimorphic in nature. A wide variety of classical genetic and modern molecular techniques are presently available to analyze these organisms, as well as the lesser well-known, but certainly not less virulent pathogens. What is important to keep in mind is the similarities between the fungi, the "big picture." Each of the individual organisms’ details can be gleaned from further readings.
2. Physiology and Taxonomy
The taxonomy of the fungi is complex, and students interested in further classification or taxonomy of fungi are referred to Ref. 1. Over the past several decades, their special place in the taxonomy of biological organisms has been reclassified from a subdivision within the plant kingdom to part of the eukaryotic world. Within the new scheme [bacteria, archea and eukarya (2)], note that the division between plants and fungi has disappeared. Even with this new assignment, there have been many proposals for their classification based upon nutritional requirements, cytology, or morphogenesis (1, 79). Perhaps the most enlightening description of the taxonomical state of the field is described by Ainsworth, "the question of the number of kinds of fungi can be more usefully approached by first considering some of the main factors which determine their recognition. H Their [fungi] recorded number and distribution is closely correlated with the number and distribution of mycologists. H Some taxonomists are perhaps unduly impressed by small differences H others, are lumpers H recognize far fewer kinds". By and large the fungi are regarded as having five phyla: oomycetes (water molds), zygomycetes (common molds), ascomycetes (sac fungi), basidiomycetes (club fungi) and deuteromycetes (imperfect fungi having no sexual stage). With the advent of recombinant DNA technology and new DNA sequencing paradigms, it will be possible to re-evaluate the relationships between certain fungi by their sequence homologies, rather than by their morphology or nutritional biochemistry. Most of the research has been centered upon a few species of the ascomycetes and basidiomyecetes although C. albicans, a member of the fungi imperfecti, has been the source of a great deal of research due to its critical role in medical mycology.
Like most of their taxonomy, fungal life cycles may vary from simple (as in the yeasts) to complex (as in the basidiomycetes). Fungi are typically non-photosynthetic heterotrophic organisms that absorb nutrients from their surrounding environment. As multicellular organisms, they display a wide range of morphological types from the microscopic yeasts and water molds to the multicellular mushrooms. They may exist as a multinucleate undifferentiated mass of cells during all or part of their life cycle, or form complex highly developed reproduction structures. In their vegetative state, fungal cells may be unicellular (yeast-like) or filamentous (mycelial or hyphal-like) in shape. Many are capable of switching between the two forms, which has been the source of a great deal of interest, especially with regard to the regulatory circuits involved and the effect on pathogenicity. All fungi are eukaryotic, but the cell may be uninucleate or multinucleate, haploid, diploid or dikaryotic during part of their life cycle. They typically contain a cell wall, which may explain their original classification within the kingdom of plants; however, the cell wall contains polymers of chitin and glucans rather than the more usual cellulose of plants.
3. Fungal Life Cycles and Reproduction
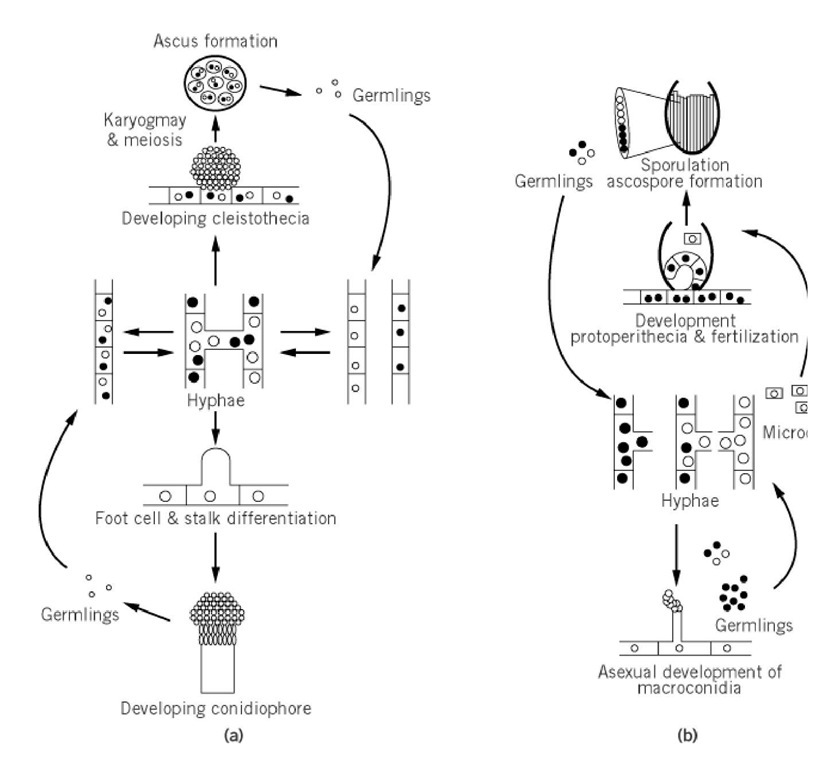
Fungi display a variety of reproductive choices. They may reproduce either asexually or sexually, or both. The fungal spore is specialized for reproduction, survival and, in some cases, dispersal. Not only are there heterothallic species such as Neurospora crassa that require different mating types in order to undergo sexual reproduction, but homothallic species (self-maters) such as Aspergillus nidulans and pseudohomothallic species that switch mating types (such as Saccharomyces cerevisiae) as well. Further, there is an assortment of fungi that do not appear to undergo sexual reproduction at all (e.g.-fungi imperfecti, including Candida albicans). The life cycles of Aspergillus nidulans and Neurospora crassa (Fig. 1) serve to illustrate many of the salient features found in this highly divergent group of organisms.
Figure 1. Schematic representation of the life cycles of Aspergillus nidulans and Neurospora crassa. (a) Aspergillus nid Asexual reproduction involves formation and maturation of conidiophores (described in the text) and uninucleate spores conidia. Vegetative haploids grow as monokaryotic or dikaryotic hyphae. These cells may then undergo sexual reproduc (upper portion of the cycle), initiated with the formation of specialized fruiting body called the cleistothecia. Eight binuc ascospores are formed within an unordered ascus. (b) Neurospora crassa. Asexual reproduction involves the formation o hyphae and the development of conidiophores, producing millions of multinucleate spores called macroconidia. Vegetati haploids grow as monokaryotic or dikaryotic hyphae. Cells of the opposite mating type are required for sexual reproduct Sporulation is initiated by the formation of the protoperitheicia, which is fertilized by microconidia of the opposite matin (see text). Cellular fusion is followed by a complex developmental pathway that involves synchronous nuclear division, karyogamy, and meiosis to yield hundreds of asci within the perithecium (see text for details).
3.1. Life Cycle of Neurospora crassa (3, 4)
N. crassa is a haploid ascomycete found in two non-switching mating types, A and a (referred to as "big A" and "little a"). Strains of either type grow as branching, thread-like cells called hyphae. These hyphal cells are typically interconnected and multinucleated. The mycelium is homokaryotic if all the nuclei present have the same genotype, and it is heterokaryotic if they have a different genetic composition. The fusion of haploids to produce diploid nuclei is rare; thus heterokaryons are naturally stable.
Certain stresses (a dry environment or carbon starvation) activates the asexual developmental pathway, which is also called conidiation. Aerial hyphae grow away from the mycelial substrate and develop a spore-producing structure that contains conidiophores. Conidiophores are chains of cells that grow by budding and develop into multinucleates spores called macroconidia. These bright orange spores are an ideal means of dispersal, as they are small (5-10 mm) and abundant.
In contrast, sexual sporulation has rather exacting nutritional requirements. It begins with the formation of protoperithecia—the female reproductive apparatus of the organism. Poorly characterized metabolic and environmental signals induce protoperithecial development in the absence of strains of the opposite mating type. In fact, more is known about the conditions that inhibit development than those that activate it. For example, protoperithecia are sparsely formed on nutritionally complex media, on submerged liquid cultures, and at temperatures above 27°C. The absence of ammonium ions seems to be critical to induce their formation, as does a marginal degree of nitrogen starvation or intense carbon deprivation. In addition, blue light is known to stimulate their formation.
Development of microconidia occurs in the absence of strains of the opposite mating type. These asexual spores are small and uninucleate, grayish-brown in color. They are formed within the vegetative hyphae and rupture through the cell wall as they mature. Microconidia are thought to function primarily as male fertilizing agents for the developing protoperithecia.
Being heterothallic, Neurospora must mate with strains of the opposite mating type to form sexual spores or ascospores. For this, trychogynes, specialized hyphal elements that emanate from protoperithecia, are fertilized by male elements (macroconidia, microconidia, or vegetative cells) of the opposite mating type. Fusion, or plasmogamy, initiates the development of the perithecium, the multicellular sexual apparatus. After plasmogamy, the male- and the female-derived nuclei coexist in a heterokaryotic tissue and divide mitotically until they are sorted into dikaryotic tissue, in which each cell compartment contains only one nucleus of each mating type. The nuclei then pair and undergo a series of synchronous mitoses until the tip of the hyphal cell in which they reside bends to form a hook-shaped cell called a crozier. There, the two nuclei undergo a coordinate mitosis yielding, after septum formation, a uninucleate basal cell, a uninucleated lateral cell, and a penultimate ascus mother cell that contains one nucleus of each mating type. It is in this cell that karyogamy, meiosis, and postmeiotic mitoses take place resulting in an ascus containing eight haploid spores in an order that reflects their lineage. About 200 such asci are generated within a typical perithecium. Once mature, the ascospores are ejected from the ascus through the ostiole in the perighecial beak. Meiotic segregation and recombination can be studied in Neurospora by analyzing individual asci or random ejected spores.
3.2. Life Cycle of Aspergillus nidulans (5-7)
A. nidulans is a homothallic ascomycete that does not require different mating types for sexual reproduction. As a vegetative mycelium A. nidulans grows via apical extension in which genetically distinct nuclei can coexist in a single thallus as heterokaryons or separately, maintaining the homokaryotic state. Although the hyphae have what appear to be individual segments, the cross walls contain pores so that the mycelium is in fact a multinucleate structure. Within the growing mycelia mat, nuclei are also capable of fusion, producing diploid thalli. This is also a relatively rare event in naturally occurring populations, but may be accomplished in the laboratory with the use of complementing auxotrophies present in each of the donor hyphae (8).
Sexual reproduction involves the formation of specialized fruiting bodies called cleistothecia containing fertile hyphae. Since A. nidulans is homothallic, mating occurs within a colony of uninucleate cells, much like wild type strains of S. cerevisiae. Nuclear fusion (karyogamy) and meiosis occur within the cleistothecia followed by the formation of ascospores within individual asci. Further differentiation of the postmeiotic nuclei involves two mitotic divisions producing binucleate ascospore progeny.
Asexual reproduction in A. nidulans involves the formation of multicellular conidiophores and uninucleate conidia. In response to signals that are as yet poorly understood, a single hyphal segment from the vegetative mycelium develops into a foot cell, which differentiates a specialized stalk and metulae. Repeated divisions of the metulae give rise to uninucleate phialides cells which undergo additional rounds of division to produce conidia. Thus, conidia are uninucleate and haploid, whereas ascospores are binucleate. A great deal of research has been applied to understanding the genetic and biochemical mechanisms that govern conidial development in A. nidulans. See Ref. 9 for review.
Although both Aspergillus nidulans and N. crassa have long genetic systems that make them ideal for study of a wide variety of eukaryotic problems, the use of recombinant DNA techniques and transformation systems in all of the fungi has enlarged the wealth of information that can be gleaned from pursuing some of the more genetically intractable organisms.
4. Genome Structure
The genomes of fungi range in size from around 7-8 Mb for Pneumocystis carinii and Ashbya gossypii to near 40 Mbp for some of the basidiomycetes (see Table 1). The presence of introns may be rare, as for C. albicans and A. gossypii, or they may be widely distributed, as for A. nidulans. It is estimated that the filamentous fungi will contain anywhere from 4500 genes in the smallest fungi to 9,000-12,000 genes in the largest genomes (Phillipsen, personal communication) (10). Because most fungal chromosomes do not condense during mitosis, their karyotypes cannot be determined by cytological methods. A size estimate may be determined utilizing pulsed-field gel electrophoresis techniques in which whole chromosomes are resolved using alternating electrical fields. Some examples are given in Table 1.
Table 1. Genome Size and Karyotype
|
Organism |
Size Number of (Mb) chromosomes |
||
|
Ashbya gossypii |
8.85 |
7 |
|
|
Aspergillus nidulans |
31 |
8 |
|
|
Candida albicans |
16- |
8-9 |
|
|
17 |
|||
|
Coprinus cinereus |
37.5 |
13 |
|
|
Cochliobolus |
35 |
1 5-1 6 |
|
|
heterostrophus |
|||
|
Histoplasma |
~31 |
7 |
|
|
capsulatum |
|||
|
Magneporthe grisea |
40 |
7 + 1-4 mini |
|
|
chromosomes |
|||
|
Neurospora crassa |
42.9 |
7 |
|
|
Pneumocystis carinii |
7-8 |
14-16 |
|
|
Saccharomyces |
13.5 |
16 |
|
|
cerevisiae |
|||
|
Schizosaccharomyces pombe |
14 |
3 |
|
|
Ustilago hordei |
18- |
16-21 |
|
|
25 |
|||
|
Ustilago maydis |
22 |
~20 |
|
The genomes of fungi are known to be somewhat plastic, as evidenced by the chromosome length polymorphisms (CLPs) observed for many different species using pulsed-field electrophoretic techniques to separate whole chromosomes by size. This polymorphism can be accounted for by both mitotic and meiotic events and is observed in both sexual and asexual fungi [reviewed in (11)].
The mechanisms by which these changes are initiated are largely unknown, but the presence of repetitive elements within the genome has been suggested to play a critical role. Reciprocal translocations have been demonstrated in two isolates of C. albicans (12) and in C. heterostrophus (13), and the expansion/contraction of tandem ribosomal DNA repeats has been documented as the cause of some CLPs in C. albicans (14), Coprinus cinereus (15) and Cladosporium fulvum (16). Subtelomeric regions of fungi are known to contain repetitive DNA sequences that have proven useful in fingerprinting individual isolates. Until recently, fungi were thought to lack transposon elements, which have been shown to influence genome fluidity in other systems. They have been identified in a number of species; a representative few are presented in Table 2. It is interesting to note that, while transposon-mediated inactivation of essential genes would, a priori, have severe consequences on single-celled systems such as S. cerevisiae, it would be of little consequence to the coenocytic lifestyles of the small and filamentous. In fact, transposons may have had significant effects upon fungal genome evolution.
Table 2. Insertional Elements in Filamentous Fungi
|
Organism |
Element |
Size |
Length of ITR (bp) |
Copy no. |
|
Nectria |
Nht1 |
2.2 bp |
_ |
0_100 |
|
haematococca |
copies |
|||
|
Fusarium oxysporum |
Fot1 |
1.9 kbp |
44 |
4_100 |
|
Botrytis cinerea |
Flipper |
1.8 kbp |
48 |
0_20 |
|
Magnaporthe grisea |
Pot2 |
1.9 kbp |
43 |
~100 |
|
MGR586 |
1.8 kbp |
42 |
0_50 |
|
|
MAGGY |
5.6 kbp |
_ |
0_100 |
|
|
Aspergillus niger |
Tan1 |
2.3 kbp |
44 |
1 |
|
Vader |
0.44 kbp |
44 |
~15 |
|
|
Cochliobolus carbonum |
Fcc1 |
1.8 kbp |
64 |
>10 |
|
Neurospora crassa |
Tad1-1 |
6.9 kbp |
_ |
~40 |
Another source of CLPs may be dispensable or "B" chromosomes. These chromosomes are not present in all members of a species, and their absence is generally thought to offer no advantage or disadvantage to the organism. Fungi, as well as other plants and animals, have small dispensable chromosomes or portions of chromosomes that may be dispensable. Their existence has been conclusively demonstrated in C. heterostrophus (13), and N. haematococca (17).
There are a number of sequencing efforts in the fungi that will shortly answer many of the questions concerning comparative genome structure (18): Aspergillus nidulans (19), Candida albicans (20), Neurospora crassa (21), and Ashbya gossypii (22) are well on their way to completion.
5. Conclusion
The past decade has been a particularly exciting time for those working on the filamentous fungi.
With the ability to transform these organisms with homologous and heterologous fragments of DNA, it has been possible to make great strides in a number of areas. Not only has there been a much greater understanding of developmental pathways, molecular clocks, and mating type in these organisms, but topics such as incompatibility, pathogenesis and evolution have been addressed at a molecular level. Furthermore, as more genomes are squenced, bioinformatics and functional genomics will play an even greater role as scientists begin to uncover the function of each of the proteins expressed in a given organism. What was once the gold of biochemists and geneticists can now be mined by molecular biologists studying human disease since fungi allow us to peek into the world of mammalian biology. Like all reviews of this nature, this one is far from complete, space limitations prevented the inclusion of many interesting topics, however excellent reviews in the field are available (a partial list is given below): transformation systems (23, 24); mating (25-28); control of gene expression (29); protein production and secretion (30-32); development and sporulation (4, 7, 8, 25, 27, 33); DNA modification strategies (34-36); fungal chromosomes and plasmids (11, 3741); incompatibility (42); signaling (43); virulence and pathogenesis (44-50); circadian rhythms (51, 52); evolution (53).