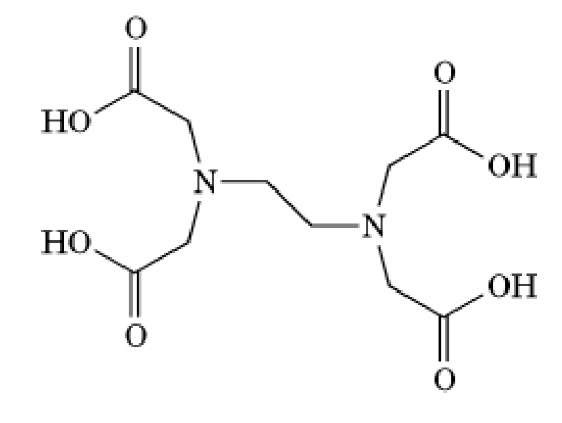
EGTA is a chelating agent widely used to control the concentration of![]() cations in biological solutions (see also -Tetraacetic Acid)). Its structure is given in Figure 1. EGTA binds
cations in biological solutions (see also -Tetraacetic Acid)). Its structure is given in Figure 1. EGTA binds![]() fold more strongly than
fold more strongly than![]() which makes it useful to control
which makes it useful to control![]() levels in the presence of physiological concentrations of
levels in the presence of physiological concentrations of![]() (1-3). The binding of cations by EGTA is pH-dependent; at low pH the carboxylate moieties will be protonated, dramatically lowering the cation affinity. The high selectivity of EGTA for
(1-3). The binding of cations by EGTA is pH-dependent; at low pH the carboxylate moieties will be protonated, dramatically lowering the cation affinity. The high selectivity of EGTA for![]() over
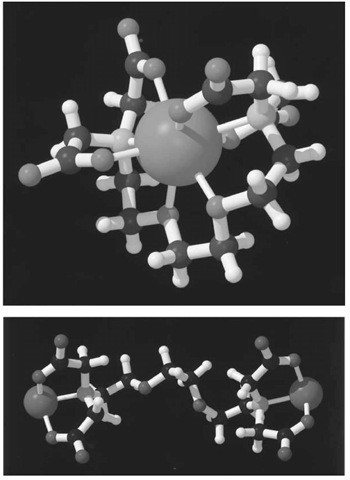
over![]() is readily explained by the structure of their respective complexes (Fig. 2, see top of next page) (4, 5). While EGTA fully wraps around
is readily explained by the structure of their respective complexes (Fig. 2, see top of next page) (4, 5). While EGTA fully wraps around![]() in a very stable octadentate complex, it cannot similarly satisfy the more stringent binding requirements of the smaller
in a very stable octadentate complex, it cannot similarly satisfy the more stringent binding requirements of the smaller![]() The preference of
The preference of![]() for oxygen ligands over nitrogen ligands is so great that it binds to EGTA via the carboxylate groups only. The remaining positions in the ligand shell are filled by water. Although these complexes can be crystallized and their structure determined, they are very dynamic in solution. NMR analysis of the
for oxygen ligands over nitrogen ligands is so great that it binds to EGTA via the carboxylate groups only. The remaining positions in the ligand shell are filled by water. Although these complexes can be crystallized and their structure determined, they are very dynamic in solution. NMR analysis of the![]() complex indicates that the carboxylate moieties interchange positions rapidly on the NMR time scale, and inversion at the nitrogen atoms is observed at elevated temperatures (5), which further indicates that the complex is very dynamic. The great stability of the
complex indicates that the carboxylate moieties interchange positions rapidly on the NMR time scale, and inversion at the nitrogen atoms is observed at elevated temperatures (5), which further indicates that the complex is very dynamic. The great stability of the![]() complex comes from the fact that all ligands are provided by a single molecule, specifically, the stability constants have a very high entropic contribution.
complex comes from the fact that all ligands are provided by a single molecule, specifically, the stability constants have a very high entropic contribution.
Figure 1. Molecular structure of EGTA.
Figure 2. Three-dimensional structures of complexes of EGTA with 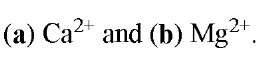
EGTA as the free acid is a white crystalline powder and has a molecular weight of 340.35 Da. The affinities of EDTA and EGTA for several cations are compared in Table 1(6).
Table 1. Comparison of Affinities of EDTA and EGTA for Various Cations3
|
Metal Ion |
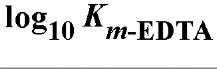 |
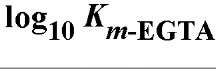 |
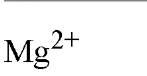 |
8.64 |
5.3 |
|
11.0 |
10.9 |
|
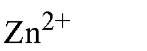 |
16.4 |
12.6 |
|
16.4 |
16.5 |
a The logarithms of their association constants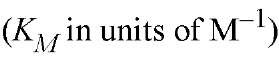 are compared at pH 7.0, 25°C,and 0.1 M ionic strength (6)
are compared at pH 7.0, 25°C,and 0.1 M ionic strength (6)