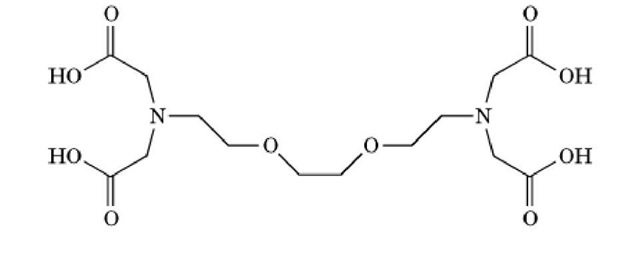
EDTA is a chelating agent widely used, along with EGTA, to control the concentration of divalent and trivalent cations in aqueous solutions. Its structure is given in Figure 1. It is an essential ingredient in such diverse products as laundry detergents and film developers. In the field of molecular biology, EDTA is present in many buffer solutions used for experiments where the presence of divalent and trivalent cations would interfere with the desired outcome. For example, it is used in most buffers for the initial steps of protein purification (1) from biological material, to suppress the activity of metal-dependent proteases that would otherwise degrade the desired protein.
Figure 1. Chemical structure of EDTA
The flexibility of the molecule, and the variety of potential liganding sites within it, permit EDTA to accommodate a wide variety of cation types, preferred ligand geometries and bond lengths, thus contributing to its high affinity for most cations (Table 1) (2). In contrast to many other complexing agents, EDTA will form only a 1:1 complex with either divalent and trivalent cations, in which a single ion is chelated by all four carboxyl groups, which greatly contributes to the stability of the resulting complex. Hence, EDTA can maintain the concentration of free cations at very low levels. The high affinity of EDTA for cations is also used to removed metals from metalloproteins and to maintain them in their metal-free state. It is important to note that the binding of cations by EDTA is pH-dependent; protonation of the carboxylate moieties at low pH dramatically lowers their affinity for cations.
Table 1. Affinity of EDTA for Various Cations®
|
Metal Ion |
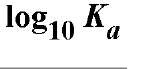 |
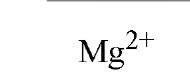 |
8.64 |
|
11.0 |
|
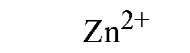 |
16.4 |
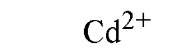 |
16.4 |
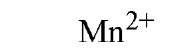 |
13.8 |
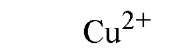 |
18.7 |
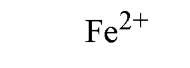 |
14.33 |
|
24.23 |
|
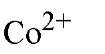 |
16.3 |
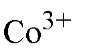 |
36 |
|
18.3 |
a The logarithm of the association constant [Ka in units of M-1 (reciprocal molars)] are compared at pH 7.0, 25°C, and 0.1 M ionic strength (2).
EDTA as the free acid is a white powder, has a molecular weight of 292.25 Da, decomposes at 240° C, and is relatively insoluble in water (0.34 g/100 mL at 25°C). The sodium and potassium salts of EDTA are also white powders and are much more readily soluble in water.