3. Self-assembly into Supramolecular Structure (Fibrillar Aggregates or Polygonal Meshworks)
3.1. Lateral Interactions Between Triple-Helical Domains
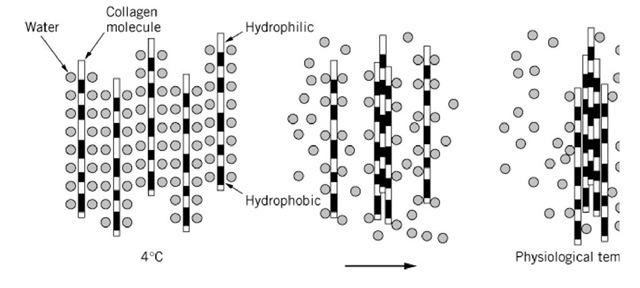
The collagen triple helix is essentially a one-dimensional molecule, with its near-constant axial separ between amino acid residues throughout the triple-helical domain. This has permitted a direct correla structural data obtained by electron microscopy with chemical sequence data. Lateral association of 1 collagenous triple-helical domains, to form fibrils, is a common feature of the collagen family. Fibril self-assemble spontaneously from solutions of extracted type I collagen when the pH, temperature, a strength are adjusted to physiological values. This lateral association appears to be promoted by elev temperature, suggesting that hydrophobic interactions are the principal driving force (Fig. 6). Fibri formation is controlled to a large extent by the amino acid sequence of the collagen and, in particular distribution of polar and hydrophobic residues that are exposed on the surface of the triple-helical do Hydroxylysine and glycosylated hydroxylysine residues might be a most potent candidate involved i limiting the lateral growth of the collagenous domains. The residues on the surface of collagen triple may well be bulky enough to cause steric hindrance in the lateral association of the triple-helical dom (Fig. 15, see later). The situation would be particularly pronounced in the triple-helical domains of ty and type IV collagens, which respectively contain more than 20 and 50 glycosylated hydroxylysine r in a single polypeptide.
Figure 6. Lateral association of triple-helical domains. Triple-helical domain is highly hydrated at 4°C. At physiological temperature such as 37°C, water molecules on the hydrophobic domains will be dehydrated. Collagen molecules accomp lateral association of the triple-helical domains through the exposed hydrophobic regions.
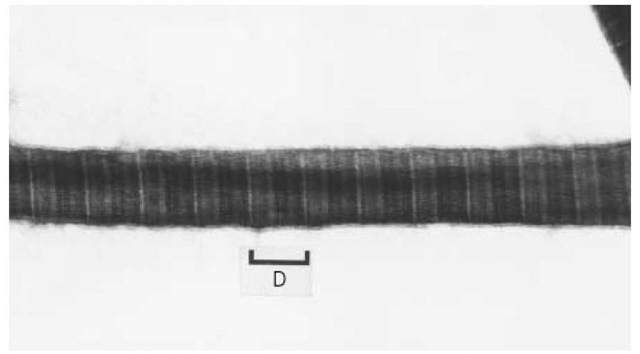
There is stringent control over lateral growth of the collagen fibrils in vivo, in that the distributions o fibrillar diameter are sharp in a wide range of extracellular matrices of various tissues. That type I cc can be organized in fibrils with different diameters may indicate the involvement of other regulatory elements. Heterotypic fibrils containing molecules of type III and type V, in addition to type I, collag molecules may predominate in vivo. The presence of the propeptide of type III procollagen, for exai the surface of collagen fibrils has led to the suggestion that this peptide may have a role in limiting tl diameter. Type V collagen was shown to form banding fibrils with finite diameter (Fig. 7). It has bee postulated that the type V collagen can form hybrid fibrils with the type I collagen. The responsible d in the type V collagen appears to be the triple-helical domain, since pepsin-treated type V collagen fo only fine fibrils. This finding suggests that type V collagen functions as one of the regulatory elemen fibril diameter, since the triple-helical domain of type V collagen forms D-period banded fibrils, with potent ability to limit the lateral growth.
Figure 7. Reconstituted type V collagen fibrils. Purified pepsin-treated type V collagen from human placenta with the ch composition of [a1(V)]2a2(V) forms the banded fibrils with finite thickness. The periodicity of the banding pattern is approximately 65 nm. Fibril thickness is 35 nm in average.
3.2. D-Staggered Lateral Association
The fibrillar structures with banded patterns are accounted for by the specific parallel and mutually staggered alignment of the triple-helical domains of the fibrillar collagens (Fig. 8). The sequence of hydrophobic and charged residues distributed along the triple helix provide maximum electrostatic and hydrophobic interactions between the neighboring molecules. The reconstituted fibrils exhibit a cross-striated banding pattern in the electron microscope, demonstrating conclusively that the aggregation of collagen molecules into axially ordered fibrillar structures is basically a self-assembly process, where the information for association is contained within the assembling molecules themselves.
Figure 8. D-staggered array of type I collagen in banded fibrils.
It has been recognized for over 30 years that the cross-striated periodic structure of the native collagen fibril is a consequence of the assembly of molecules in a parallel array, but mutually staggered (ie, axially displaced with respect to one another) by approximately one-quarter of their length—often, which is referred to as the quarter-staggered array. The periodicity of the cross-striations in the fibril is explained by the fact that each collagen monomer has eight highly charged regions 67 nm apart that appear under appropriate conditions as stained bands.
The banded period (the D period) is confirmed by low-angle X-ray scattering of rat-tail tendon fibrils, and the overall length of a fibrillar collagen monomer is 4.4 D units (when D = 234 amino acids, the length of one cross-striation period is 67 nm). Within a collagen fibril, the molecules are staggered by integral multiples of the distance D (Fig. 8).
3.3. Collagen Supramolecular Aggregates in Tissues
The organization and arrangement of the collagen fibrils and polygonal meshwork in tissues are subject to considerable variation. Fibrillar collagen, type I collagen in particular, provides the major mechanical strength of skin, tendon, bone, dentine, cornea, sclera, and so on. The fibrillar collagen types form long, unbranched, banded fibrils with a characteristic periodicity of 60-70 nm. The fibrillar aggregate of collagen is classified as a quasi-crystal, because of its highly symmetrical insoluble structure built up of essentially identical subunits. Especially in tendon, type I collagen accounts for approximately 90-95% of the dry weight of the tissue and is present as large, highly orientated fibers. All the fibrillar units are arranged in large parallel bundles, with the average diameter of the fibrils varying between 50 and 500 nm. In dermis, where type I collagen accounts for 80-90% of the collagenous proteins, the fibrils form a coarse network partially oriented in the plane of the skin, with the average diameter of the fibrils between 40 and 100 nm. The orthogonally arranged and precisely packed fibrils in corneal stroma are of particular interest, since the arrangement may well be related to tissue transparency. In the cornea, which has a high content of type V and type I collagens, the collagen fibrils have a uniform diameter of approximately 25 nm. Concentric circles of collagen fibrils in cortical bone provides another type of organization and arrangement.
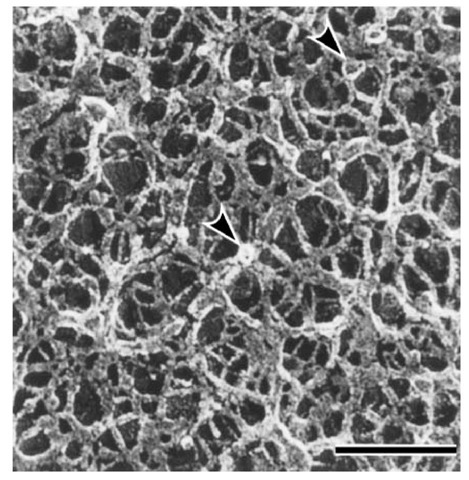
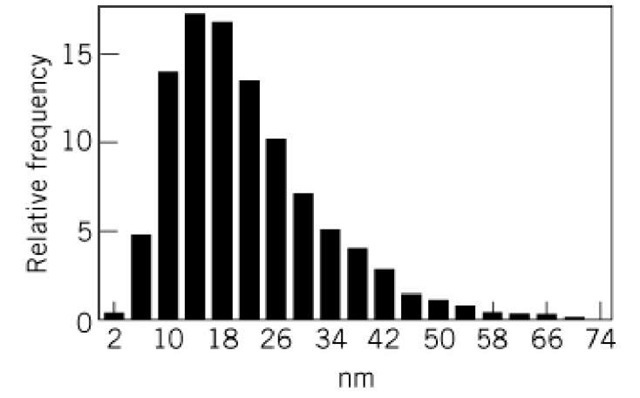
The skeletal structure of the basal lamina has a special three-dimensional meshwork with an average pore size of about 18 nm. Isolated type IV collagen can reconstitute into polygonal meshwork (Fig. 9) with an average pore size also of about 18 nm (Fig. 10). This suggests that the self-assembled supramolecular structure of the type IV collagen is formed primarily through the lateral interactions of the triple-helical domain, which has kinks or bending points due to interruptions of the Gly—X —Y repeat.
Figure 9. Polygonal meshwork structure of type IV collagen aggregates. Bar in lower right corner is equal to 100 nm. Arrowheads indicate globules presumably formed through NCl domains.
Figure 10. Histograms of the distances between two branching points of polygonal meshwork of type IV collagen aggregates.
4. Biosynthesis and Chain Assembly of Collagen
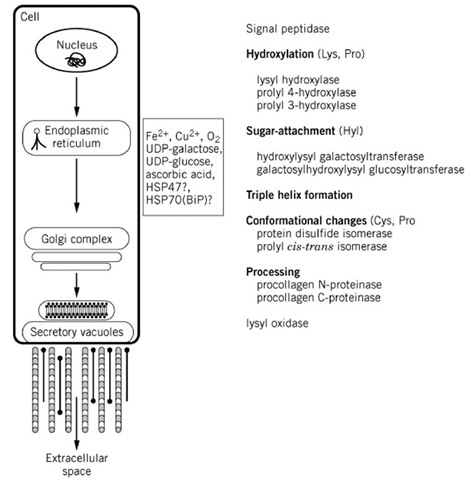
Biosynthesis of the collagen molecule is a complex process that begins with transcription of the individual collagen genes and culminates in the chains assembled into collagen molecules (Fig. 11). This biosynthetic process involves a number of co- and posttranslational modifications unique to collagenous sequences. Intracellular modifications of the newly synthesized polypeptide chains result in the formation of triple-helical molecules. At least 10 different enzymes have been implicated in the posttranslational processing of the collagen molecule. The biosynthesis of type I collagen can be regarded as a useful model exemplifying many of the common features of collagen biosynthesis. The nonfibrillar collagens may, however, deviate from the general scheme.
Figure 11. Schematic drawing of the complex process of biosynthesis of collagen. Collagen translation products are directed into the endoplasmic reticulum by the N-terminal signal peptide that is the cleaved from the polypeptide chain by signal peptidase. Individual procollagen chains undergo complex enzyme-catalyzed post-translational modifications prior to the completion of chain assembly and the folding of the triple helix. Hydroxylation of Lys and Pro and attachment of sugars to hydroxylysine (Hyl) are characteristic post-translational modifications of collagenous polypeptides.
4.1. Pre-procollagen mRNA Translation
The pre-proa collagen chains that are the primary translation product contain hydrophobic N-terminal signal sequences of 22-26 amino acid residues, similar to those in most other secreted proteins and are essential for targeting of nascent polypeptides to the endoplasmic reticulum (see Protein Secretion). During or shortly after translocation, the signal sequence is removed by the signal peptidase on the luminal side of the endoplasmic reticulum membrane.
Hydroxylation of proline and lysine residues of the newly synthesized collagen polypeptide chains results in the formation of procollagen molecules containing an array of hydroxyproline and hydroxylysine residues. This hydroxylation is produced by collagen-specific enzymes within the cell
4.2. Helix Formation and Secretion
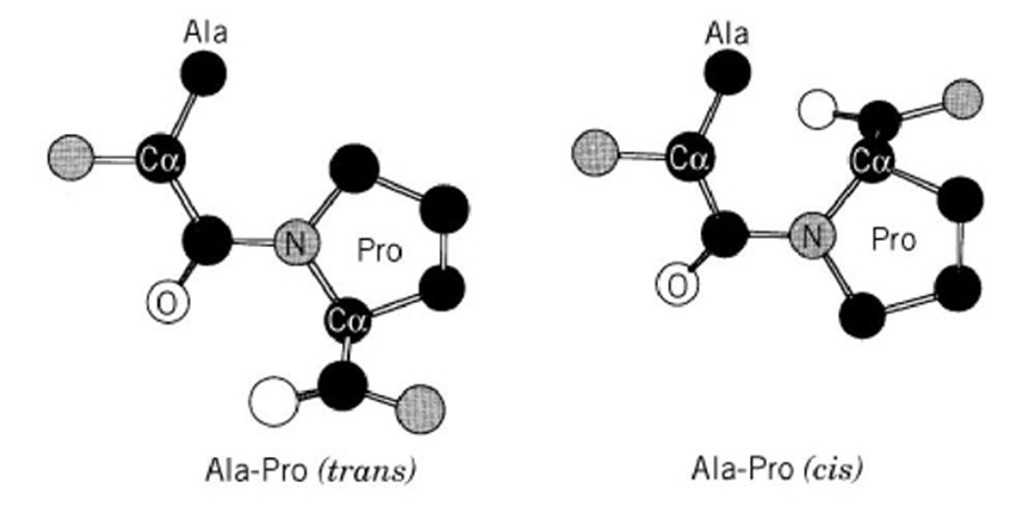
The assembly of three polypeptides into a triple-helical collagen molecule is a complex process initiated by association of the three C-terminal propeptides (in the case of type I collagen), which involves chain alignment, nucleation, and propagation into the triple-helical conformation. Requiring the C terminus of the polypeptide chain, chain association and alignment (in the case of type I collagen) takes place only as the chains near full elongation, and folding may proceed in the C- to N-terminal direction. Association of the C termini is stabilized by disulfide bond formation catalyzed by the enzyme protein disulfide isomerase (PDI). Assembly of the triple helical molecule might be limited by cis-trans isomerization (Fig. 12) of the peptide bonds preceding proline residues, which is catalyzed by a cytosolic enzyme, peptidylprolyl cis-trans isomerase (PPI). Formation of the triple-helical conformation appears to preclude further enzymatic post-translational modifications. The procollagen in a triple-helical conformation is ready to be secreted into the extracellular space.
Figure 12. Cis-trans isomerization of Ala-Pro peptide bonds. H atoms are not shown.
Stable triple helix formation requires 4-hydroxyproline in the Y position of a high proportion of —Gly—X—Y— triplets (at least 100 of the 1000 amino acids of the helical domain of type I collagen) (see Hydroxylation (Lysine, Proline)). The normal rate of procollagen secretion is apparently dependent on triple-helical conformation. If triple helix formation is prevented by inhibiting prolyl hydroxylase (by the use of a,a’-dipyridyl or by limiting the availability of the cofactors Fe or O2), the nonhelical proa(I) chains first accumulate within endoplasmic reticulum. 4.3. Extracellular Polymerization
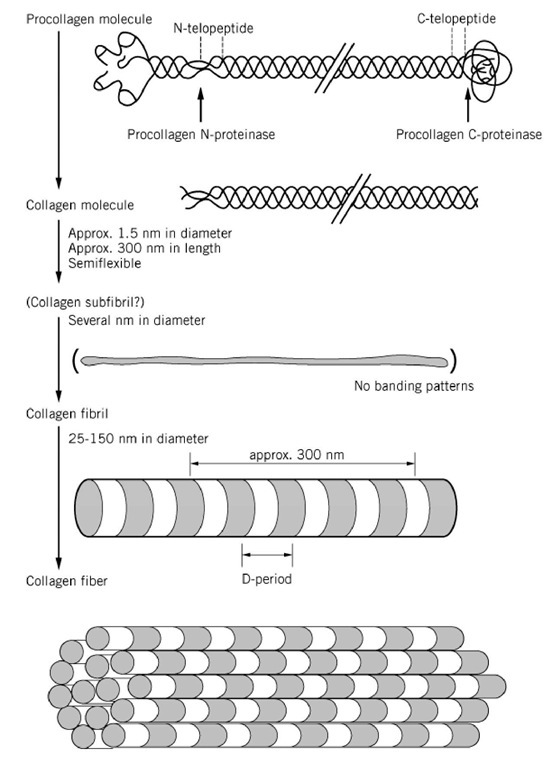
Fibril formation involves the specific enzymatic cleavage of the procollagen N- and C-terminal propeptides in the extracellular space (Fig. 13), and these enzymes must therefore play a key role in regulating the aggregation. Such processing is characteristic of the fibrillar collagens and may not be a universal prerequisite for the assembly of all collagen types. The cleavage of the N- and C-terminal propeptides from the procollagen I molecule at specific peptide bond cleavage sites is achieved by two specific neutral metalloproteinases—procollagen N-proteinase and procollagen C-proteinase— both of which require Ca for activity and are inhibited by metal chelators. These enzymes act essentially only on molecules in the triple-helical conformation. Type I procollagen N-proteinase cleaves the N-terminal propeptides of types I and II procollagens between a proline and a glutamine residue. A separate enzyme is involved in the processing of the N-propeptide of type III procollagen. The type I procollagen C-terminal proteinase cleaves the C-terminal propeptides of both the proa1(I) and proa2(I) procollagen chains at an Ala—Asp bond. Most recently, procollagen C-proteinase was found to be BMP-1(bone morphogenetic protein-1), and recombinant BMP-1 was shown to act as procollagen C-proteinase. The enzyme is also able to cleave the C-terminal propeptides of type I procollagen homotrimer, type II procollagen, type III procollagen, and other proteins, including laminin-5 and prolysyl oxidase.
Figure 13. Processing of type I procollagen to fibril formation.
4.4. Crosslinking of Type I Collagen in the Fibrils
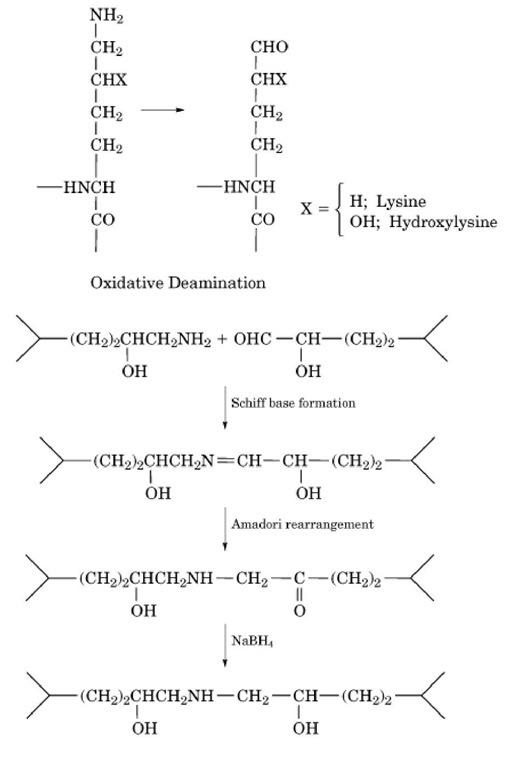
Covalent crosslinking of type I collagen in the fibrils involves oxidative deamination of specific lysine or hydroxylysine residues in the nonhelical (telopeptide) regions at the ends of the triple helix. Thus, lysyl and hydroxylysyl aldehydes are generated by lysyl oxidase (protein lysine 6-oxidase) (Fig. 14). The catalytic activity of lysyl oxidase is dependent on strict steric requirements (in the case of type I collagen, the quarter-stagger arrangement of molecules in the fibril) and on the sequence of amino acids surrounding the "target" lysyl/hydroxylysyl residues. The recombinant enzyme was produced as a proenzyme, which could be processed for activation by BMP-1 or procollagen C-proteinase.
Figure 14. Oxidative deamination and crosslinking of lysine or hydroxylysine residues. The oxidative deamination, Schiff base formation, and Amadori rearrangements take place in vivo. The last step, treatment with sodium borohydride, is carried out in the laboratory to stabilize the crosslinks for chemical analysis.
The majority of covalent crosslinks stabilizing the fibrillar collagens involve the telopeptides and, consequently, pepsin treatment of insoluble, crosslinked fibrils tends to release the triple-helical domain, which can be recovered in its native conformation.
5. Posttranslational Modifications of Proline and Lysine Residues
Hydroxylation of proline and lysine residues is a post-translational modification that is specific to collagen (see Hydroxylation (Lysine, Proline)). The reason for hydroxylation of proline residues at the 3 position is not known, but the presence of an appropriate number of 4-hydroxyproline residues in the —Y— position of—Gly—X—Y— is a crucial determinant of the stability of the triple helix under physiological conditions. The hydroxyl group forms hydrogen bonds that contribute to the thermal stability. The transition temperature (thermal denaturation temperature; melting temperature, Tm) of triple helices formed in vitro by nonhydroxylated collagen is about 25°C, which is 15°C lower than the fully hydroxylated collagen triple helix (Fig. 3). The importance of hydroxylysine may be inferred from the inherited connective tissue disorder, Ehlers-Danlos syndrome type VI, in which lysyl hydroxylase deficiency results in impaired crosslink formation and consequent susceptibility to mechanical disruption of tissues.
5.1. Glycosylation of Hydroxylysine Residues
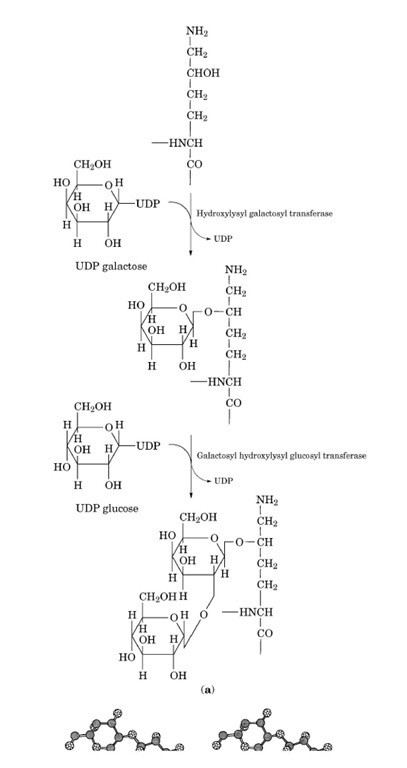
Vertebrate collagen molecules contain the monosaccharide galactose and the disaccharide glucosyl-galactose. The glycosides are covalently linked through the hydroxyl group to hydroxylysine residues within the triple-helical domains (see Figs. 15 and 16).
Figure 15. Glycosylated hydroxylysine residues on the surface of the collagen triple helix. The molecular model of a portion (36 residues/chain) of human type V collagen, [a1(V)]2a2(V), was built up by the McMolw (Molecular Images Software, San Diego, CA, USA) and Chem3D (CambridgeSoft Corporation, Cambridge, MA, USA) programs, using the 2CLG file of the Protein Data Bank, which is the theoretical model of the (Gly—Pro—Hyp)4 trimer. The two lysine residues of the two a1(V) chains are either left unchanged (left) or changed to glulcosylgalactosylhydroxylysine (right). The glycosylated hydroxylysine residues are evidently bulkier than other residues.
Figure 16. Attachment of galactose and glucose groups to hydroxylysine residues of collagen. (a) Enzymatic steps in coupling the two sugars; (b) stereo view (direct) of glucosylgalaclosylhydroxylysine. H atoms are not shown.
This glycosylation is unique to collagen, and its extent is variable both between collagen types and within the same collagen in different tissues and at different ages. A potential biological role of the specific carbohydrate units would be the regulation of the lateral assembly of the triple-helical domains. An inverse relationship exists between carbohydrate content and collagen fibril diameter, suggesting that the steric hindrance to the formation of highly ordered fibrils might be caused by the bulky glycosylated hydroxylysine residues. The glycosylation of hydroxylysine residues within the triple helix proceeds through the action of two isolated enzymes: hydroxylysyl galactosyl transferase (UDP galactose: 5-hydroxylysine-collagen galactosyltransferase) and galactosyl hydroxylysyl glucosyl transferase (UDP glucose: 5-hydroxylysine-collagen glucosyltransferase). Galactose is transferred to hydroxylysine residues by the former enzyme, while glucose is transferred to galactosyl-hydroxylysine residues by the latter enzyme. The carbohydrates are provided in both reactions by the appropriate UDP-glycosides. Both enzymes require a divalent cation (preferably Mn ) for the activity. A free e-amino group in the substrate hydroxylysyl residue and a nonhelical polypeptide conformation are requirements for both transferases. The reactions are more favorable with longer peptides. Hydroxylysyl galactosyl transferase can bind at least two manganese ions per molecule of active enzyme.
![Reconstituted type V collagen fibrils. Purified pepsin-treated type V collagen from human placenta with the ch composition of [a1(V)]2a2(V) forms the banded fibrils with finite thickness. The periodicity of the banding pattern is approximately 65 nm. Fibril thickness is 35 nm in average. Reconstituted type V collagen fibrils. Purified pepsin-treated type V collagen from human placenta with the ch composition of [a1(V)]2a2(V) forms the banded fibrils with finite thickness. The periodicity of the banding pattern is approximately 65 nm. Fibril thickness is 35 nm in average.](http://what-when-how.com/wp-content/uploads/2011/05/tmp2152_thumb2_thumb.jpg)
![Glycosylated hydroxylysine residues on the surface of the collagen triple helix. The molecular model of a portion (36 residues/chain) of human type V collagen, [a1(V)]2a2(V), was built up by the McMolw (Molecular Images Software, San Diego, CA, USA) and Chem3D (CambridgeSoft Corporation, Cambridge, MA, USA) programs, using the 2CLG file of the Protein Data Bank, which is the theoretical model of the (Gly—Pro—Hyp)4 trimer. The two lysine residues of the two a1(V) chains are either left unchanged (left) or changed to glulcosylgalactosylhydroxylysine (right). The glycosylated hydroxylysine residues are evidently bulkier than other residues. Glycosylated hydroxylysine residues on the surface of the collagen triple helix. The molecular model of a portion (36 residues/chain) of human type V collagen, [a1(V)]2a2(V), was built up by the McMolw (Molecular Images Software, San Diego, CA, USA) and Chem3D (CambridgeSoft Corporation, Cambridge, MA, USA) programs, using the 2CLG file of the Protein Data Bank, which is the theoretical model of the (Gly—Pro—Hyp)4 trimer. The two lysine residues of the two a1(V) chains are either left unchanged (left) or changed to glulcosylgalactosylhydroxylysine (right). The glycosylated hydroxylysine residues are evidently bulkier than other residues.](http://what-when-how.com/wp-content/uploads/2011/05/tmp2160_thumb2_thumb.jpg)