This method of peptide mapping involves generating of protein fragments via limited proteolysis, usually in the presence of SDS, followed by electrophoretic separation in SDS-PAGE. The resulting one-dimensional peptide patterns are characteristic of the protein substrate and are used to evaluate structural relationships between proteins and to aid identification of individual polypeptide chains. The technique is named after the first author of a 1977 publication describing the procedure (1), and many variants of this method have subsequently been presented (2).
After initial separation and excision from a primary gel, the protein substrate is digested in solution or, typically, in the stacking zone of the SDS-PAGE "mapping gel." The solution approach relies on purifying the protein by conventional chromatographic procedures or preparative SDS-PAGE followed by excision and electroelution from the gel (3). Protein preparation in SDS-containing gels is feasible because the subsequent digestion is performed in the presence of SDS. Frequently, SDS-PAGE isolation of the protein is possible at an early stage of purification, which is important when only a small amount of the protein is available. For Cleveland mapping, the efficient approach is to place the gel slice that contains the protein band of interest directly into a well of a second SDS/polyacrylamide gel and to add a suitable proteolytic enzyme. After electrophoresis just long enough for the protein substrate to migrate out of the gel piece and enter the stacking zone with the proteinase, the power is temporarily switched off and the gel left for digestion to take place. When the power is turned on again, the fragments of the protein substrate are separated from the proteinase and are mapped in the resolving gel. This procedure is preferred when handling small amounts of protein because of the direct transfer between primary and secondary gels.
The original Cleveland protocol (1) involved brief staining of the primary gel with Coomassie Brilliant Blue, followed by rapid destaining and excision of the band corresponding to the protein substrate. Because fixation of the protein is undesirable due to the electrotransfer, negative staining with 1 M KCl may be an alternative and has been used for subsequent electroblotting and sequence analysis (4). The excised gel piece is equilibrated in buffer containing 0.1% (w/v) SDS to unfold the protein substrate and to obtain a suitable pH of 6.8 for stacking gel electrophoresis and digestion (1). Often, 5 to 10 |ig of protein substrate is required for application onto the primary gel to generate a proteolytic pattern detectable by Coomassie Blue staining, but the minimum amount can be substantially lowered if silver staining fluorescence techniques or radiolabeling are used to visualize the peptide map.
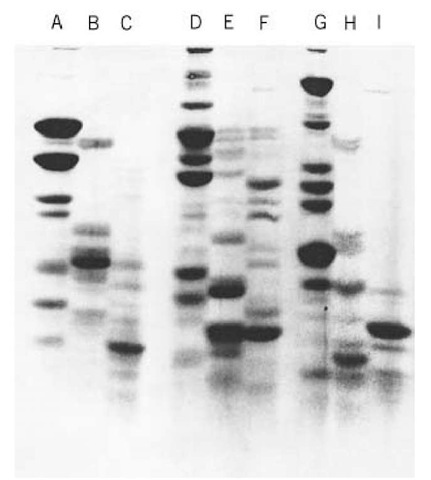
After equilibration, the gel piece that contains the protein is placed in a well of the "mapping gel," and, in the original protocol, overlaid with a solution of the appropriate proteinase in the same buffer. In later protocols, loading of the proteinase below the gel piece is suggested to avoid a variable depth of enzyme solution around the gel piece (2). Once a narrow stack has formed after the initial period of current, usually when the tracking dye (e.g., bromphenol blue) is approaching the bottom of the stacking gel, the power is turned off for 30 to 60 min. This is normally sufficient for the proteinase to cleave susceptible peptide bonds, which typically generates 10 to 20 fragments of 50- to 70-kDa proteins (1). It is crucial that the proteolytic enzyme is active in 0.1% SDS (up to 0.5% for in-solution digestions). Suitable enzymes are trypsin, a-chymotrypsin, and papain, in addition to the popular V8 proteinase from Staphylococcus aureus. All are added in proportions corresponding to an enzyme to substrate ratio in the range of 1:10 to 1:50 (w:w). After digestion, electrophoresis is resumed, and the resulting fragments are resolved, typically in a 15 or 20% acrylamide gel of the discontinuous type (Fig. 1). A unique proteolytic pattern is obtained for each protein substrate and for each individual proteinase. This discriminating feature makes Cleveland mapping useful as a tool for visually judging the similarity between proteins.
Figure 1. One-dimensional Cleveland maps of albumin (A,D,G), tubulin (B,E,H), and alkaline phosphatase (C,F,I), generated by cleavage with papain (A-C), Staphylococcus aureus V8 proteinase (D-F), and chymotrypsin (G-I), followed by SDS-PAGE in a 20% (w/v) acrylamide gel. Each combination of protein substrate and proteinase gives a unique pattern.
In addition to enzymes, specific chemical cleavage is employed where the protein substrate within a gel is treated in a test tube. Suitable reagents are cyanogen bromide for cleavage after methionine residues, #-chlorosuccinimide for cleavage at tryptophan, hydroxylamine for cleavage at Asn-Gly peptide bonds, and formic acid for cleavage at Asp-Pro bonds. As for the size of the protein substrates, the method is generally applicable to polypeptide chains in the range 10 to 100 kDa.
Peptide fragments smaller than 10 kDa are difficult to resolve into informative peptide maps, although separating such small peptides is possible by using different SDS-PAGE systems. An alternative is to recover small fragments separately by size exclusion chromatography for subsequent peptide mapping by reverse-phase HPLC. This can be useful also for proteins large than 10 kDa in order not to waste small fragments (5).