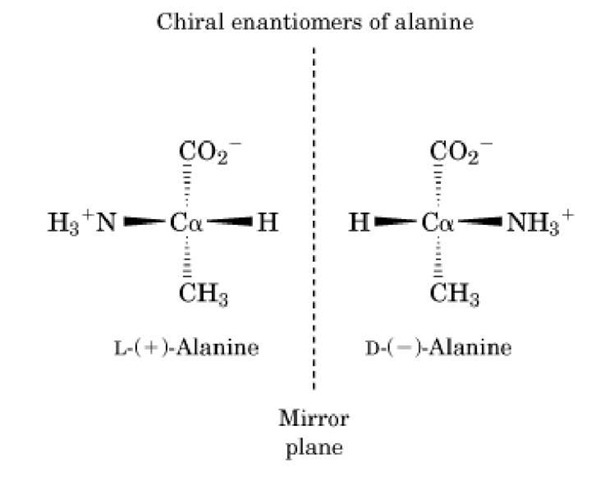
The word chiral is derived from the Greek word "cheir" for "hand." It was first used to describe any molecule that could not be superimposed on its mirror image, like left and right hands, by Lord Kelvin (1). This supplanted the less precise term dissymetrical used by Pasteur (2). The nonsuperimposable mirror image isomers are enantiomers. The physical property that differentiates enantiomers is the direction that they rotate plane polarized light. Thus, the two enantiomers are differentiated as either dextro(+) or levo(-) rotatory, depending on whether the rotation of the polarized light is clockwise or counterclockwise, respectively. Solutions containing an excess of one of the enantiomers are said to be optically active. A solution containing exactly equal concentrations of both enantiomers is called racemic (see Racemic And Racemization).
The most common structural element that generates chiral molecules is the presence of a tetrahedral atom with four different substituents, by definition a chiral center. The a-carbon of the a-amino acids (Fig. 1), except for glycine, and the hydroxymethylene carbons of carbohydrates are all chiral centers. The mirror images of chiral molecules have different absolute configurations, which are uniquely identified by the rules proposed by Cahn et al. (3) (see Configuration).
Figure 1. The enantiomers of alanine, which are mirror images. Note that the a-carbon is chiral because of the four different substituents. The (l) and (d) designate the absolute configuration of the chiral center, whereas the (+) and (-) indicate which direction an aqueous solution of the enantiomer will rotate the plane of polarized light.
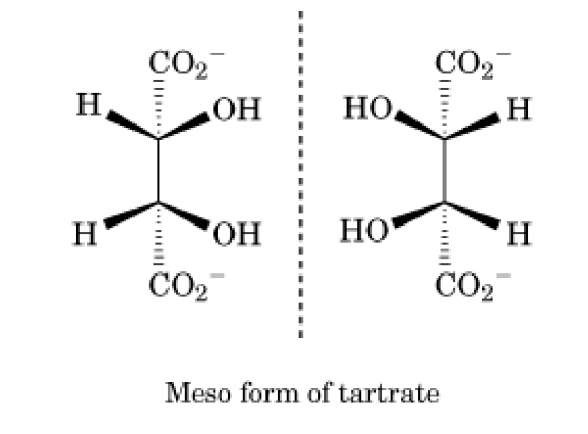
Possessing a tetrahedral chiral center is not required for chirality, nor is the presence of a chiral center proof that the molecule will be chiral. Other arrangements of atoms can generate nonsuperimposable mirror images. One example of biochemical importance is the hexacoordinate complexes of metal ions, such as the Mg ATP complex (4). Other chiral molecular structures have been surveyed by Testa (5). A molecule with an internal mirror plane may have chiral centers but not be chiral. These are meso compounds. Because of their internal symmetry, these molecules can be superimposed on their mirror image. The diastereomeric form of tartaric acid, which has two chiral centers, but a mirror plane between C2 and C3, provides an example of a meso compound (Fig. 2).
Figure 2. Meso tartrate, which contains two chiral centers C2 and C3, but is not chiral because there is an internal plane of symmetry. Note that, unlike the enantiomers of alanine, these mirror images can be superimposed by a 180° rotation.