B-DNA is the predominant type of DNA structure under physiological conditions. The B-DNA double helix is right-handed, with its base pairs perpendicular to the helix axis that passes through the center of the base pairs. The major groove and minor groove are roughly equivalent in depth, 8.5 and 7.5 A, respectively, whereas the width of the major groove is ~12 A and that of the minor groove is ~6 A.
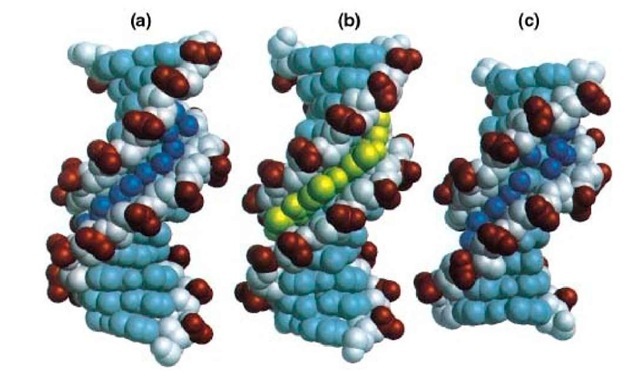
The first X-ray crystallography structure of B-DNA (Fig. 1A) was that of a dodecamer sequence d (CGCGAATTCGCG)2 that contains the EcoRI restriction sequence GAATTC (1). There are a number of interesting structural features associated with this B-DNA structure. A distinctive feature is that the minor groove is narrower at the AATT region of the double helix than the CGCG ends. The narrow minor groove at the AATT region is filled by a spine of water molecules that form hydrogen bonds to both the O2 of thymines and the N3 of adenines, although this spine of waters has been reinterpreted recently using a higher resolution structural analysis as a spine of waters on sodium ions (2). Additionally the base pairs in the central AATT region of the helix have high propeller twist angles. The propeller twisting enhances the stacking of the bases along each strand of the double helix, and the AT base pairs are in general less restrictive to propeller twisting because of fewer hydrogen bonds than the GC base pairs (3). Lastly, the sugar pucker of the deoxyribose ring favors the C2′-endo conformation, although a range of conformations from C1′-exo to O4 ‘-endo is also present. This may reflect the greater flexibility associated with B-DNA structures.
Figure 1. Examples of the structure of B-form DNA. (a) Crystal structure of the double-stranded dodecamer d (CGCGAATTCGCG) (Protein Data Base BDL084), with the water molecules in the minor groove shown as spheres. (b) Crystal structure of the double-stranded d(CGCAAATTTGCG)-distamycin A complex (Protein Database GDL003). The drug displaces the waters in the minor groove. (c) Crystal structure of the double-stranded decamer d (CCAGGCCTGG) (Protein Database BDJS30), with the waters bound to the minor groove shown as spheres.
The narrow minor groove region associated with the A-T sequences in B-DNA affords an excellent binding site for the minor groove-binding drugs, such as distamycin A, netropsin, Hoechst 33258, Hoechst 33342, and DAPI. The crystal structure and solution structures of the complexes of several DNA oligonucleotides with those minor groove-binding drugs, most of them in 1-1 drug-to-duplex complexes (Fig. 1B), have been determined (4, 5). Those structures revealed that the drug replaces the spine of hydration in the narrow minor groove and stabilizes the DNA structure, without perturbing the overall conformation significantly. The narrow minor groove associated with the central AATT base pairs is essential for the 1-1 binding mode of drugs. The binding energy derives in part from the gain in entropy associated with the displacement of the water molecules. The sequence preference for A-T regions by minor groove-binding drugs is due to the greater negative electrostatic potential at the bottom of the minor groove at A-T regions. Finally the presence of N2 amino groups of guanines provides both a charge and a steric hindrance to drug binding.
NMR study of the binding of distamycin A (a pyrrole-containing anti-tumor antibiotic) to DNA containing A-T sequences revealed a more complex pattern. Distamycin A can bind DNA not only in a 1-1 drug/duplex complex but also in a 2-1 mode, with two distamycins bound to the minor groove in an antiparallel, side-by-side manner (6). The latter binding mode requires that the minor groove width at the binding site be expanded so as to accommodate two drug molecules, reflecting the flexibility of B-DNA. This new finding has stimulated an active study in the design of new minor groove-binding compounds that can bind to all four base pairs, (A-T, T-A, C-G, and G-C), with high specificity. It was found that compounds with imidazole-containing units do not have a great discriminating power regarding the recognition toward G/C versus A/T base pairs. However, compounds that combine the imidazole units with the pyrrole units can be designed to possess excellent sequence-specific binding properties. Rules for such designs have been proposed recently (7).
Additional work on the high-resolution crystal structures of several DNA decamer oligonucleotides with "mixed" sequences, including d(CCAGGCCTGG) (Fig. 1C), showed that the minor groove width of those structures in general is wider, but with some variations. Those DNA decamers have a slightly narrow or wide minor groove, depending on the sequence. In general, the A-T regions have a narrower minor groove than the G-C regions. The hydration structure in the groove is dependent on the groove width. It was noted that a single spine of water molecules along the floor of the minor groove is associated with a narrow minor groove, whereas a ribbon of double water molecules, bridging the base edge N or O atom to the O4′ atoms of the sugar ring, is associated with the wide minor groove.