The abl gene was first identified as the transforming element of Abelson murine leukemia virus (A-MuLV), a replication-defective retrovirus that was isolated after inoculating Moloney murine leukemia virus (M-MuLV) into prednisolone-treated BALB/c mice (1). A-MuLV induces B-cell lymphomas in vivo and transforms both lymphoid and fibroblastic cells in vitro. The proviral genome of A-MuLV encodes a single polypeptide chain that is a fusion product of the virally-derived gag and cell-derived abl sequences (2). Sequence analysis of c-abl sequences revealed that, like the src gene, the abl gene codes for a tyrosine kinase that also contains the unique, SH3, SH2, and tyrosine kinase domains (3). Unlike the src gene, however, the abl gene product contains an additional C-terminal domain whose function is not entirely clear. In addition, the gene product of c-abl does not contain the negative-regulatory tyrosine residue at its C-terminus. The tyrosine kinase activity of the c-Abl protein is negatively regulated by its SH3 domain, which is deleted from the v-abl gene product. Interestingly, it was also found that the v-abl gene product contains a point mutation in its C-terminal sequence, which enhances its tyrosine kinase and transforming activities (4). Thus, both the v-src and v-abl genes have alterations in their regulatory sequences that result in the constitutive activation of their tyrosine kinase activities, which correlates with their transforming function.
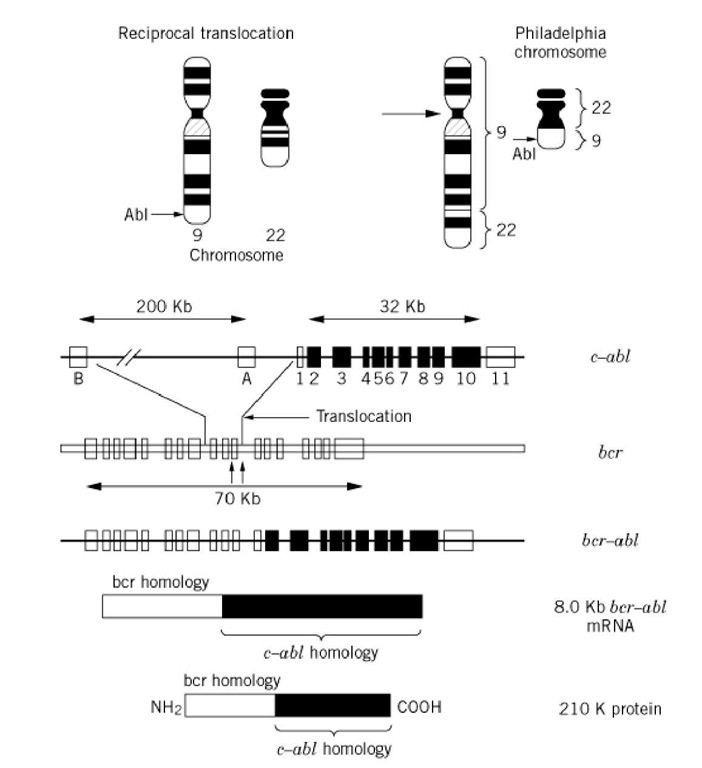
Gene mapping studies have established that the c-abl oncogene is located on human chromosome 9q34, the location where the break point occurs in the Philadelphia chromosome. The Philadelphia chromosome is generated when a portion of the c-abl gene is translocated to chromosome 22 and is fused to a portion of the gene called bcr, which itself is disrupted during the translocation process (Fig. 1). This process results in generating a new gene, called bcr-abl, that has enhanced oncogenic activity and whose expression leads to the development of leukemia (5, 6). It is interesting to note that the BCR-ABL gene is structurally similar to the gag-abl gene encoded by the Abelson murine leukemia virus. Both the gag-abl and bcr-abl genes exhibit high levels of tyrosine kinase activity, which is essential for their transforming activity.
Figure 1. Generation of the bcr-abl oncoprotein by chromosomal translocation. The abl gene in a normal cell, is located on chromosome 9 and encodes a tyrosine kinase. During malignant transformation of myeloid cells, a portion of chromosome 9 that contains the abl locus translocates to chromosome 22 at the breakpoint cluster region (bcr) locus and generates the chimeric bcr-abl oncoprotein. Because the translocation results in deleting the sequences that negatively regulate abl tryosine kinase activity, the fusion protein has constitutive and increased levels of enzymatic activity.
The function of c-abl in normal cell growth is not fully established. The c-abl gene codes for two 145-kDa proteins as a result of alternative splicing of the two first exons (see RNA Splicing and Introns, Exons). This results in synthesizing two proteins that differ in their amino-terminal sequences. Both forms of c-Abl are found in the cytoplasm and in the nucleus. The c-Abl protein can bind to DNA and to cell-cycle regulators like the retinoblastoma protein. Recent studies show that c-abl gene expression is induced during cellular stress caused by agents, such as ionizing radiation and certain other genotoxic agents. These agents induce the formation of a complex involving c-Abl, DNA-dependent protein kinase, and Ku antigen (7). The DNA-dependent protein kinase in this complex is activated by DNA damage and in turn phosphorylates and activates c-Abl. Recent studies have also shown that c-Abl associates with the product of the ATM gene, which in turn activates c-Abl in response to ionizing radiation. DNA damage also induces binding of c-Abl to p53 and contributes to cell-cycle arrest at the G1-phase, which is mediated by p53. Recent studies also show that the c-Abl protein binds to protein kinase C-S and phosphorylates the latter, resulting in its activation and translocation to the nucleus, where it participates in inducing apoptosis (8). Thus, a substantial amount of evidence gathered in the past few years indicates that c-Abl protein has a pivotal role in mediating cellular growth arrest and the apoptotic effects that occur during exposure to ionizing radiation and genotoxic stress.