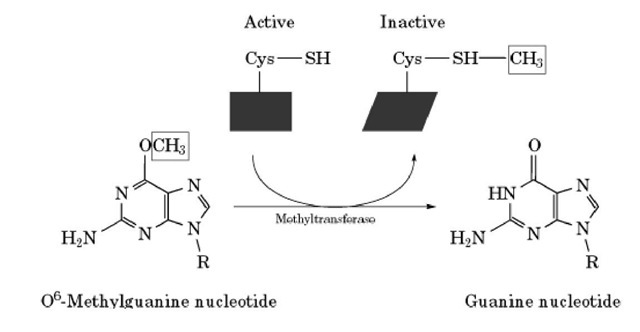
MGMT is an important enzyme of DNA repair. It carries out direct repair of alkylated DNA by transferring the alkyl group from oxygen atoms within the DNA molecule to a cysteine residue on the enzyme. This reaction is irreversible, and thus the MGMT is said to be a "suicide" enzyme (Figure 1). The three lesions repaired by MGMT are O6-methylguanine, O4-methylthymine, and methylphosphotriesters induced by methylating agents such as N-methyl- N’-nitro-N-nitrosoguanidine (MNNG). The enzyme also repairs other alkyl guanines, however, such as O6-ethylguanine and O6-butyl-guanine, albeit at a much lower efficiency.
Figure 1. The "suicide mechanism" reaction catalyzed by MGMT. The enzyme transfers a methyl group from the O6 position of guanine to the active-site cysteine residue of the MGMT polypeptide chain to restore the normal base and form the stable S-methylcysteine adduct, which inactivates the enzyme.
MGMT has been found in all free-living species tested. Enzymes from these sources exhibit considerable sequence homology and contain the active-site cysteine residue within the -Pro-Cys-His-Arg-Val-signature sequence. Escherichia coli contains two MGMTs: Ada and Ogt. The Ada protein was the first MGMT discovered, and it mediates the "adaptation" response in this organism (1): Exposure to subtoxic doses of an alkylating agent such as MNNG renders cells resistant to subsequent toxic doses of the same or related alkylating agents. Ada is a protein of 39 kDa, and it has two well-defined, and easily separable, NH^- and COOH-terminal domains. It performs three functions (2): (i) positive transcriptional regulator, (ii) phosphotriester methyltransferase, and (iii) O 6-methylguanine methyl transferase. The active-site residue Cys69 in the N^-terminal domain accepts methyl residues from the phosphodiester backbone, to form S-methyl-cysteine. Methylation of Cys69 causes a conformational change in Ada and enables it to activate the promoters of ada, alkA (methyladenine DNA glycosylase), alkB, and aidB genes and thus to increase cellular resistance to alkylating agents. The COOH-terminal domain contains the active-site Cys321, which carries out direct repair by accepting methyl groups from O6-MeGua and O4-MeThy, to form S-methyl-cysteine and to restore the normal bases. In addition to Ada, E. coli contains another MGMT called Ogt (3). This enzyme is structurally and functionally homologous to the COOH-terminal domain of Ada; it is expressed constitutively and is not part of the adaptive response.
Like E. coli, most other bacteria tested exhibit the adaptive response, although the mechanistic details might vary (see DNA Damage, Inducible Responses To). In Bacillus subtilis, AdaA has methylphosphotriester methyltransferase and transcriptional activator activities, in a manner analogous to the NH^-terminal domain of Ada of E. coli (4). The AdaB of B. subtilis, similarly carries out the MGMT function performed by the COOH-terminal domain of the E. coli Ada protein. In B. subtilis there is also a noninducible MGMT (Ada C) comparable to the E. coli Ogt protein (4).
The eukaryotic MGMTs (including the human enzyme) are structural and functional homologues of Ogt-type methyltransferases (Mr ~20kDa); that is, they are suicide enzymes that repair O6- methylguanine and O4-methylthymine (5). They have no transcriptional regulatory function, nor is there evidence that these enzymes are induced by DNA damage specifically. In humans, increased resistance to anticancer alkylating agents occurs when there is a loss of MGMT activity by gene silencing, accompanied by a mismatch-repair defect arising from a mutation in a mismatch-repair gene.