Instrumental SMR Conditioning (ISC) and its Impact on Sleep Quality and Declarative Learning
Recently we applied the earlier described IEC method to investigate the effect of instrumental 12-15Hz (SMR) conditioning (experimental group) as compared to randomized IEC (control group) on sleep as well as declarative memory performance (Berner, Schabus, Wienerroither, & Klimesch, 2006; Hoedlmoser et al., 2008).
In a pilot study done in our laboratory (Berner et al., 2006) we investigated whether (i) ISC can have direct influence on sleep spindles produced during the night and whether (ii) a subtle change in this activity could even have an impact on successful memory encoding or overnight memory consolidation. These questions were hypothesized according to earlier findings where we indicated a significant positive correlation between overnight change in the number of recalled words in a declarative word-pair association task and spindle activity change from a control to experimental night (Schabus et al., 2004). Although sleep spindle activity remained unchanged after ISC in this first study, we found enhanced "spindle frequency" band power during sleep, indicating that ISC effects become most easily evident in the actual trained frequency bands rather than in associated phasic spindle "events". The caveat of this pilot study, however, was the amount of ISC-sessions: the ISC-protocol consisted only of four 10min long blocks, thus, was not comparable to the much more intense ISC-protocols used in previous studies.
Inspired by this preliminary pilot study we continued our investigations by a more extensive approach. Twenty-seven healthy subjects (13 male, 14 female; mean age = 23.63 years, SD=2.69) were randomly assigned (parallel group design) to either (i) a SMR-conditioning protocol (experimental group; N=16) or to (ii) a randomized IEC protocol (control group; N=11). As depicted in Figure 2 all of them attended the laboratory on 13 occasions.
Figure 2. Study design. Subjects had to attend the laboratory 13 times. The first visit – 3 days prior to pretreatment – served as entrance examination. Pretreatment included a declarative memory task (encoding [ENCpre], retrieval before nap [RET1pre], retrieval after nap [RET2pre]) as well as a 90min nap (NAPpre) and was followed by 10 IEC sessions on 10 consecutive days (except weekends). Posttreatment (same procedure like pretreatment: ENCpost, RET1post, NAPpost and RET2post) – one day after the last conditioning session – completed the study protocol.
First subjects had to pass an entrance examination consisting of several parts: clinical evaluation of sleep quality [Pittsburgh Sleep Quality Index (PSQI); Buysse, Reynolds, Monks, Berman, & Kupfer, 1988]; anxiety (self-rated anxiety scale; Zung, 1971); depression (self-rated depression scale; Zung, 1965); memory („Wechsler Memory Scale – revised"; Harting, Markowitsch, Neufeld, Calabrese, & Deisinger, 2000) and intelligence (Advanced Progressive Matrices; Raven, Raven, & Court, 1998). Throughout the study participants had to complete a sleep diary every day in the evening and in the morning (Self-rating scale for Sleep and Awakening quality; Saletu, Wessely, Grunberger, & Schultes, 1987) to control their sleep-wake-cycle and to prevent sleep deprivation prior to laboratory examination. During the nap-session before (NAPpre) and after (NAPpost) ISC subjects performed a declarative word-pair association task (Plihal & Born, 1997). Subjects had to learn a first list of 80 word-pairs during NAPpre, and a second one during NAPpost. Polygraphic sleep recordings using Synamps EEG amplifiers (NeuroScan Inc.) started at 2:00 pm and ended at 3:30 pm. Fifteen gold-plated silver electrodes were attached according to the international 10/20 system. In addition, four electrooculogram (EOG) channels, one submental EMG channel, one electrocardiogram channel (ECG) and one respiratory channel (chest wall movements) were recorded. Between pre- and post-treatment the subjects were trained to enhance their band amplitude within specific frequency bins during 10 IEC sessions on 10 consecutive days (except weekends) using visual online feedback. Each session was conducted in a standardized procedure and lasted for about 1 hour (including electrode adjustment). Immediately before and after instrumental conditioning subjects were instructed to relax during a 2min eyes-closed resting condition followed by a 2min eyes open resting condition. EEG was recorded from C3 with reference on the right earlobe and ground electrode placed on the left earlobe. For offline artefact rejection a bipolar vertical EOG channel was recorded. The ongoing EEG at site C3 was band-pass filtered to continuously extract amplitude values within the frequency of interest. Band amplitude of interest was calculated online and used as relevant conditioning parameter. The instrumental conditioning design was performed as depicted in Figure 3. One trial consisted of a 3sec baseline followed by a continuous feedback interval lasting until the EEG signal exceeded the predefined reward threshold measured during the baseline for more than 250ms. Any time the subject was able to produce the requested EEG rhythm, the compass needle moved to the left. The aim was to move the needle as far to the left as possible reaching the previously fixed threshold represented by a green dot. In case of exceeding the amplitude threshold for at least 250ms, subjects got an audiovisual reward (appearance of a sun for 2sec accompanied by a 200ms lasting sound of 800Hz).
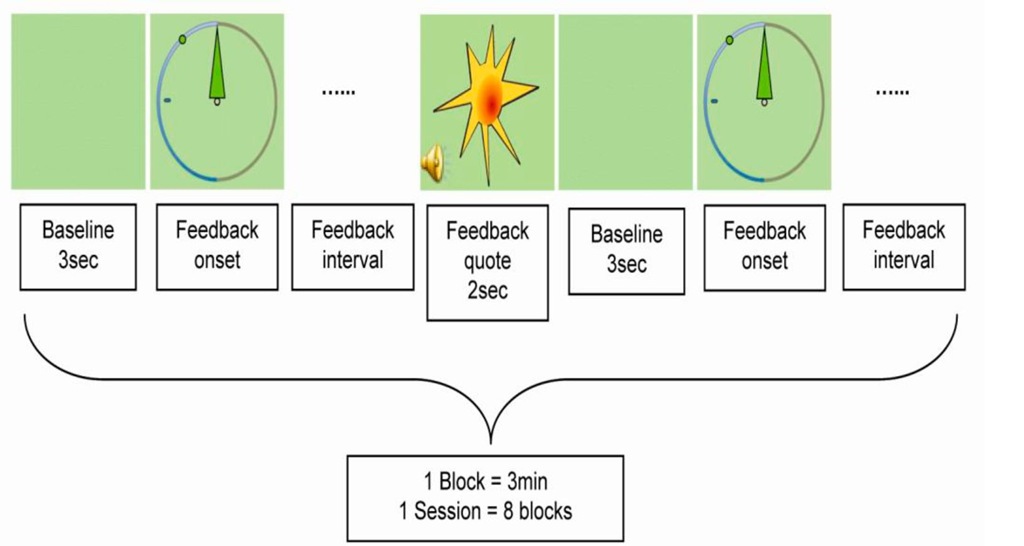
Figure 3. Schematic representation of one block within an IEC session. A 3sec lasting "Baseline" before visual feedback onset was used to calculate the mean amplitude within the frequency of interest which served as reference during "Feedback interval". An audiovisual "Feedback quote" was triggered by an EEG signal containing at least 250ms of the frequency of interest at an amplitude exceeding a certain reward threshold measured during the 3sec baseline.
There were no specific instructions for the subjects as everybody was encouraged to find his or her own appropriate strategies like physiological relaxation combined with positive mental activity. To prevent rewards elicited by movements, eye, or muscle artefacts, trials with amplitudes exceeding 200^V were abandoned by starting a new trial. Experimental and control group only differed concerning frequency adjustments. Subjects of the experimental group had to enhance the amplitude within their 12-15Hz frequency range throughout all sessions, whereas for the control group the band amplitude of randomized, each session varying 3Hz frequency bins between 7 and 20Hz (except 12-15Hz) were used as conditioning parameter. Subjects were not aware about their treatment until the study was over.
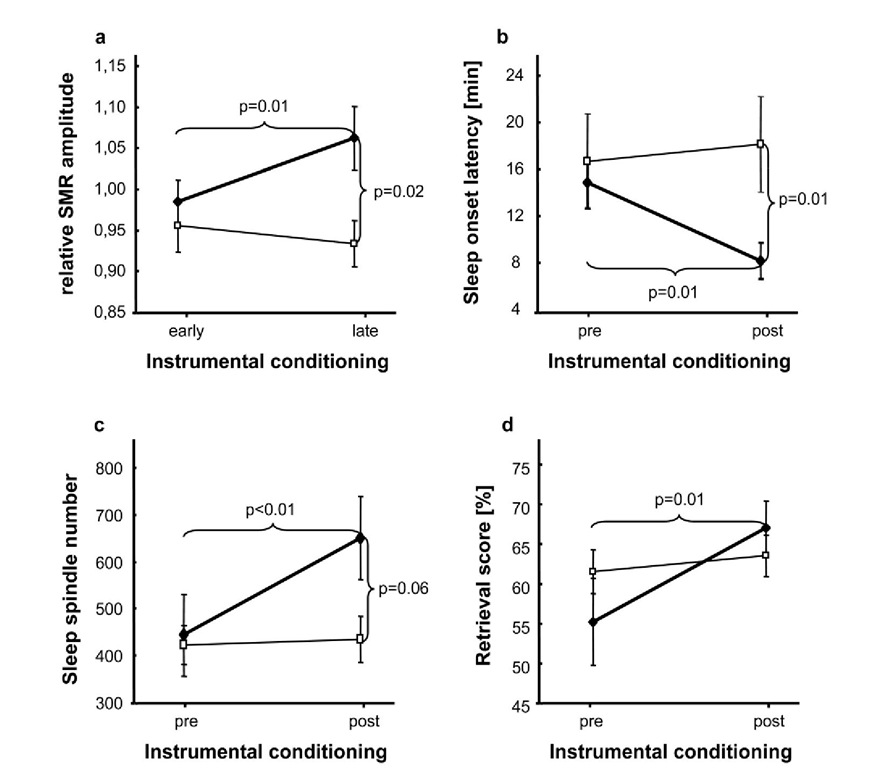
Figure 4. Main effects of ISC compared to randomized IEC on sleep and learning. 2-way ANOVAs depicting differences between experimental (♦) and control (□) group, a Significant increase of relative SMR amplitude after ISC (experimental group) vs. randomized IEC (control group). b Significant reduction of sleep onset latency during NAPpost compared to NAPpre. c Significant increase of sleep spindle number from NAPpre to NAPpost. d Significant enhancement of retrieval score computed at immediate cued recall (RET1) after ISC (experimental group) compared to randomized IEC (control group). Note that only 1215Hz conditioning (experimental group) could increase relative SMR amplitude, sleep spindle number and retrieval score as well as decrease sleep onset latency. Error bars indicate standard errors of mean.
As depicted in Figure 4a significant SMR amplitude changes from early to late conditioning sessions confirmed the success of our ISC paradigm. Most interestingly, these EEG changes transferred into sleep (Figure 4b-c) and even improved immediate memory retrieval after learning (Figure 4d). There were no effects on memory consolidation (i.e., "overnap" change in memory performance after ISC) indicating a more unspecific effect of ISC. Heightened attention or relaxation levels after ISC are supposed to cause the improvement in word-pair recall. Our results therefore demonstrate to our best knowledge for the first time successful ISC in a healthy human population (cf. Figure 4a) leading to enhancement of sleep spindles (cf. Figure 4c) and thereby indicating that specific neural mechanism trained during wakefulness can be translated into sleep. There was no change in the duration of stage 2 sleep and therefore it can be ruled out that the spindle increase is caused by an increase of stage 2 sleep. Furthermore, sleep onset latency (cf. Figure 4b) was significantly shortened after ISC compared to a randomized IEC paradigm. Therefore our results additionally support Hauri’s approach (1981, 1982) to use ISC as an alternative treatment for primary insomnia. Note that here we used a much more rigorous control group than usually adopted. Subjects of the control group underwent exactly the same study protocol with only the type of IEC being altered.
Prospects: ISC as Treatment for Primary Insomnia?
Given our recent findings (Hoedlmoser et al., 2008) we further aimed at changing sleep quality in humans suffering from primary insomnia by using ISC.
In a preliminary study we recruited 12 subjects (11 women) aged between 19 and 48 (M =29.33; SD = 10.56) with clinical symptoms of primary insomnia. Individuals suffering from primary insomnia had to meet the following inclusion criteria: a) difficulty initiating [i.e., sleep-onset latency (SOL), >30min] and/or maintaining (i.e., time awake after sleep onset >30min) sleep and b) insomnia or its perceived consequences caused marked distress or significant impairment of occupational or social functioning. Several questionnaires [PSQI, Buysse et al., 1989; Becks Depression Inventory (BDI-II), Beck et al., 1996; Becks Anxiety Inventory (BAI), Margraf & Ehlers, 2007)] as well as a semi structured clinical interview for sleep disorders ("Strukturiertes Interview fur Schlafstorungen nach DSM-III-R" (SIS-D), Schramm et al., 1993) were used to evaluate subjects compatibility (primary insomnia without comorbidities).
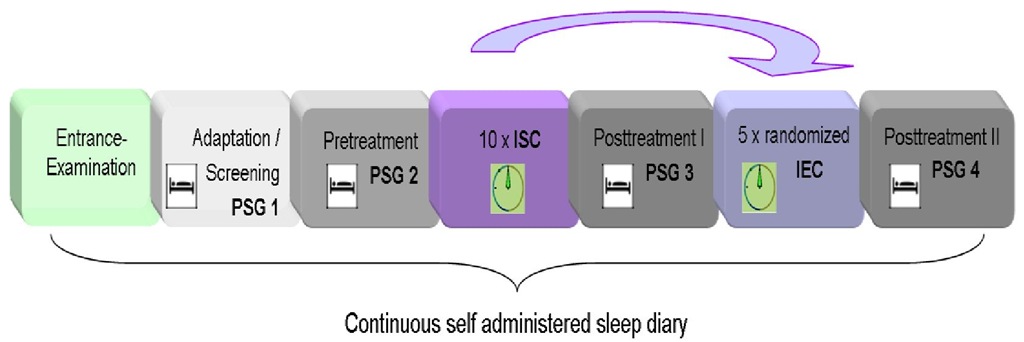
A counterbalanced within subjects design was used. Subjects had to attend the sleep laboratory 19 times over the course of 3 to 6 weeks (4 nights, 10 x ISC, 5 x randomized IEC; cf. Figure 5). Participants were instructed to arrive at the sleep laboratory at 7:30 pm each night. Polysomnographic recordings (PSG) started between 11:00 and midnight and were terminated after eight hours time in bed (TIB). The first PSG night served as screening and adaptation night. During experimental nights (1 x pre-treatment, 2 x posttreatment) a standard PSG montage with 21 gold plated silver electrodes was applied.
Concerning ISC we used the same method like in our previous study (Hoedlmoser, 2008) however, this time a within subjects design was implemented. Subjects were randomly assigned to either a protocol starting with ISC followed by randomized IEC or to a protocol starting with randomized IEC followed by ISC. Therefore every subject received both treatments – ISC and randomized IEC (cf. Figure 5).
Preliminary results confirmed the increase of 12-15Hz activity over the course of the ten ISC training sessions (p=0.027) but not over the course of the randomized IEC training sessions. Interestingly, the increased SMR activity was associated with the enhancement of subjective sleep quality measured by the PSQI (p=0.001). Furthermore sleep onset latency was tendentially reduced after ISC (p=0.056) but not after randomized IEC. However, there were no significant changes concerning sleep spindle activity like we could show in our previous study (Hoedlmoser et al., 2008).
Figure 5. Study design. Subjects suffering from primary insomnia take part in a first adaptation/screening night (PSG1) followed by an experimental pre-treatment night (PSG 2). Pre-treatment night was either followed by 10 ISC sessions or by 5 randomized IEC sessions. Note that the order of treatments (ISC or randomized IEC) was counterbalanced. Post-treatment nights (PSG3, PSG4) were each conducted one day after the last conditioning session of ISC or randomized IEC, respectively.
Therefore our current preliminary data indicate that people suffering from primary insomnia experience subjective benefits from ISC before objectively verifiable. Further research is highly needed in order to reveal whether more intense ISC – or other forms of biofeedback or neurofeedback are able to consistently improve also objective sleep parameters such as wake after sleep onset or total sleep time.
Taken together our recent work confirms that instrumental conditioning of SMR has positive impact on sleep quality as well as on declarative learning in healthy participants (Hoedlmoser et al., 2008). Whether a similar instrumental conditioning protocol is also effective in the treatment of sleep disorders such as primary insomnia has yet to be revealed by well-controlled empirical studies.
![Study design. Subjects had to attend the laboratory 13 times. The first visit - 3 days prior to pretreatment - served as entrance examination. Pretreatment included a declarative memory task (encoding [ENCpre], retrieval before nap [RET1pre], retrieval after nap [RET2pre]) as well as a 90min nap (NAPpre) and was followed by 10 IEC sessions on 10 consecutive days (except weekends). Posttreatment (same procedure like pretreatment: ENCpost, RET1post, NAPpost and RET2post) - one day after the last conditioning session - completed the study protocol. Study design. Subjects had to attend the laboratory 13 times. The first visit - 3 days prior to pretreatment - served as entrance examination. Pretreatment included a declarative memory task (encoding [ENCpre], retrieval before nap [RET1pre], retrieval after nap [RET2pre]) as well as a 90min nap (NAPpre) and was followed by 10 IEC sessions on 10 consecutive days (except weekends). Posttreatment (same procedure like pretreatment: ENCpost, RET1post, NAPpost and RET2post) - one day after the last conditioning session - completed the study protocol.](http://what-when-how.com/wp-content/uploads/2012/04/tmp115120_thumb.jpg)