Biofeedback
Biofeedback is a technique in which people learn how to self-control certain internal bodily processes that normally occur involuntarily, such as heart rate, blood pressure, muscle tension, skin temperature and brain activity. To provide biofeedback special computer hardware is required. Biofeedback instruments are supposed to i) monitor a physiological process of interest, ii) measure what is monitored and finally iii) present what is measured as meaningful information by translating the raw signal e.g., into a tone that varies in pitch, a visual meter that varies in brightness, or a computer screen that varies the lines moving across a grid. The patients have to find mental strategies to self-control the parameter of interest. Through trial and error participants learn to identify and control their mental activities that will bring about the desired physical changes. The three most commonly used forms of biofeedback are i) electromyography (EMG; muscle tension) biofeedback, ii) thermal biofeedback (skin temperature) and iii) EEG biofeedback (neurofeedback; brain activity). Concerning biofeedback as treatment for sleep disorders there are two types of biofeedback that have been effectively used: EMG and EEG biofeedback. According to the "Practice Parameters for the Psychological and Behavioral Treatment of Insomnia" by Chesson et al. (1999) and Morgenthaler et al. (2006) biofeedback is an effective and recommended therapy in the treatment of chronic insomnia. It has been rated as probably efficacious indicating that multiple observational studies, clinical studies, wait list controlled studies, and within subject and intrasubject replication studies demonstrated efficacy. However, after receiving considerable attention in the 1970s and 1980s (cf. Table 3) there has been a big gap until today – only one study of biofeedback treatment for insomnia has been published in the last 20 years (Sanavio et al., 1990). This lack of research interest may be related to the fact that biofeedback requires more time than comparable forms of relaxations therapies with only little appreciable advantage.
Additionally commercial hardware for detecting and managing psychophysiological parameters are needed and the biofeedback operator should understand the fundamental principles and methods for detecting and measuring physiological processes in depth. Despite this lack of research, biofeedback seems to be an efficacious treatment for insomnia. It is also to note that after the intense and promising research in the 1970s many people in the field applied the method rapidly to a variety of clinical disorders (e.g., migraine headache, anxiety disorders, epilepsy, tinnitus, attention deficit (hyperactivity) disorder, chronic pain) thereby skipping the much needed basic research documenting biofeedback efficacy convincingly. Therefore our starting point was to vigorously and objectively test the EEG biofeedback (or neurofeedback) methodology by conducting a highly controlled study using sensorimotor (12-15Hz, SMR) "instrumental conditioning" with the aim to influence brain patterns during waking as well as sleep.
Table 3. Overview of literature concerning Biofeedback and Insomnia.
Non-Pharmacological Alternatives for the Treatment of Insomnia
Abbreviations:![]() = increase;
= increase;![]() = decrease; WASO = wake after sleep onset; CBT = cognitive behavioral therapy; PSG = polysomnography; EMG = electromyography; NFT = neurofeedback-training;
= decrease; WASO = wake after sleep onset; CBT = cognitive behavioral therapy; PSG = polysomnography; EMG = electromyography; NFT = neurofeedback-training;
SMR = sensorimotor rhythm; EEG = electroencephalography
Instrumental EEG Conditioning (IEC)
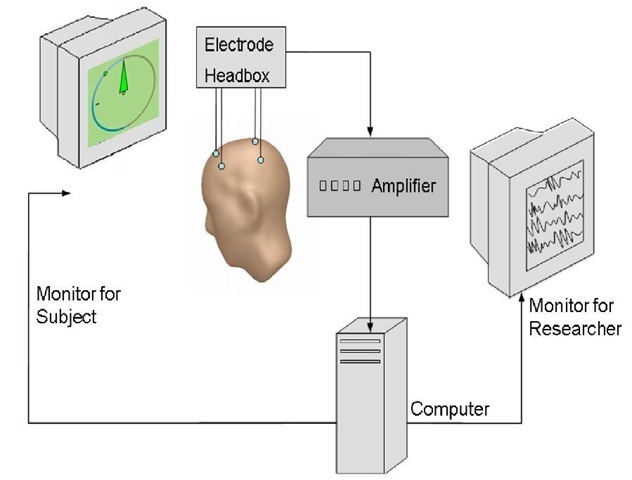
Instrumental conditioning of EEG parameters – often called neurofeedback or EEG biofeedback – is a very sophisticated type of biofeedback and refers to an operant conditioning paradigm (for review see Budzynski, Budzynski, Evans, & Abarbanel, 2009; Sterman, 1996). Participants are instructed to learn to self regulate distinct parameters of their cortical activity (e.g., amplitude, frequency or coherence) as assessed by the means of EEG. The aim of IEC is to teach individuals what specific states of cortical arousal feel like and how to activate such states voluntarily. During IEC – as depicted in Figure 1 – EEG is recorded and the relevant components are extracted and "fed back" to the individual using an online feedback loop (audio, visual or combined audio-visual). The individual’s task may then be to increase/decrease the respective cortical parameter. When the correct EEG-pattern is produced, the subject receives a positive response or reward by the computer.
Figure 1. Equipment requested for IEC. Scalp electrodes capture brain oscillations and transmit them to the amplifier. After amplification signals are transmitted to the computer where online calculations (e.g., Fast Fourier Transformation) are performed. Pre-processed data are presented to the subjects either visually (e.g., compass-needle) and/or acoustically (e.g., varying tone pitches). During IEC subjects permanently get realtime feedback of the parameters (e.g., SMR band power) intended to be changed (e.g., by relaxing). Additionally the researcher can supervise the session by a separate monitor.
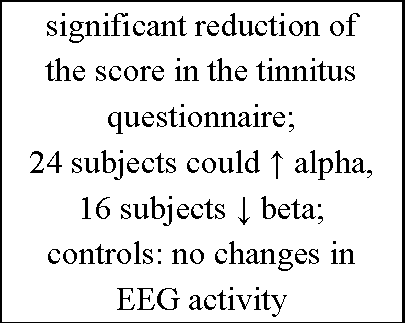
It is proposed that IEC is not successful below 10 training sessions (Egner & Gruzelier, 2003) and there is a very high variability (from 1 up to 50) in the number of sessions used for training throughout literature (cf. Table 4). After an initially enormous research interest in the 60ies and 70ies and a dramatic decrease thereafter a kind of "Neurofeedback Renaissance" seems to take place. Today IEC is mainly used as a therapeutic tool to treat different types of disorders like epilepsy (Lantz & Sterman, 1988; Kotchoubey, Strehl, Holzapfel, Blankenhorn, Froscher, & Birbaumer, 1999; Sterman & Lantz, 2001; Strehl et al., 2006; for review see Sterman & Egner, 2006; Tan et al., 2009) or attention-deficit hyperactivity disorder (ADHD; Beauregard & Levesque, 2006; Fuchs, Birbaumer, Lutzenberger, Gruzelier, & Kaiser, 2003; Gevensleben et al., 2009; Kaiser & Othmer, 2000; Leins et al., 2007; Lubar, Swartwood, Swartwood, & O’Donnell, 1995; Lubar, Swartwood, Swartwood, & Timmermann, 1995; Strehl, Leins, Goth, Klinger, Hinterberger, & Birbaumer, 2006; for review see Arns, de Ridder, Strehl, Breteler, & Coenen, 2009; Heinrich et al., 2007; Monastra, 2005). Within the treatment of other clinical disorders such as depression (Rosenfeld, Baehr, Baehr, Gotlib, & Ranganath, 1996; for review see Hammond, 2005), tinnitus (Gosepath, Nafe, Ziegler, & Mann, 2001; for review see Dohrmann et al., 2007), anxiety (Hardt & Kamiya, 1977) and substance abuse (Moore, Trudeau, Thuras, Rubin, Stockley, & Dimond, 2000; for review see Peniston & Kulkosky, 1999; Sokhadze et al., 2008) IEC has also been reported to be a useful instrument. Furthermore, the exciting progress since the pioneering work of Nicolelis (for review see Nicolelis, 2003) in the field of brain-computer or brain-machine interface enabling "locked-in" and partly paralysed patients to communicate or to produce movements, respectively, by voluntarily controlling neuronal activity (Birbaumer et al., 1999; Hinterberger et al., 2004; Pfurtscheller, Muller, Pfurtscheller, Gerner, & Rupp, 2003; for review see Birbaumer & Cohen, 2007; Birbaumer, Ramos Murguialday, Weber, & Montoya, 2009) benefits from this specific method.
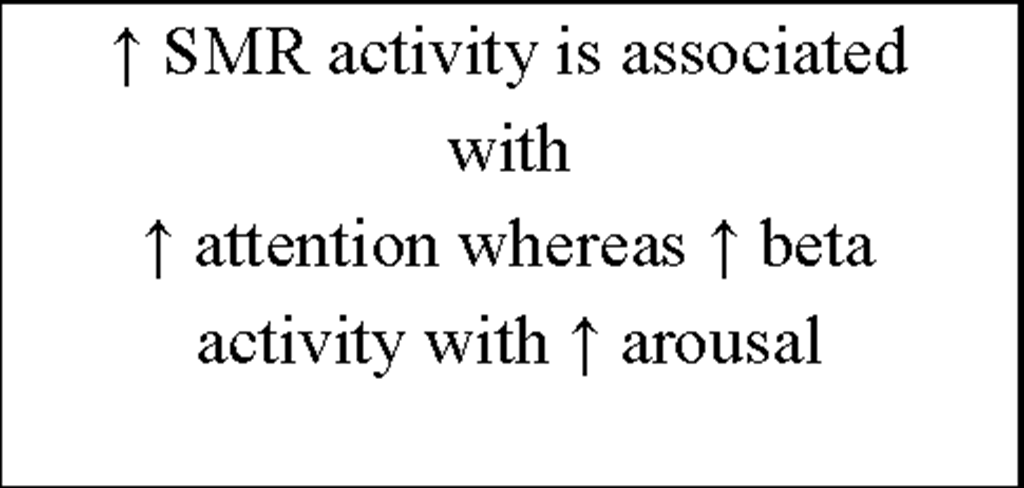
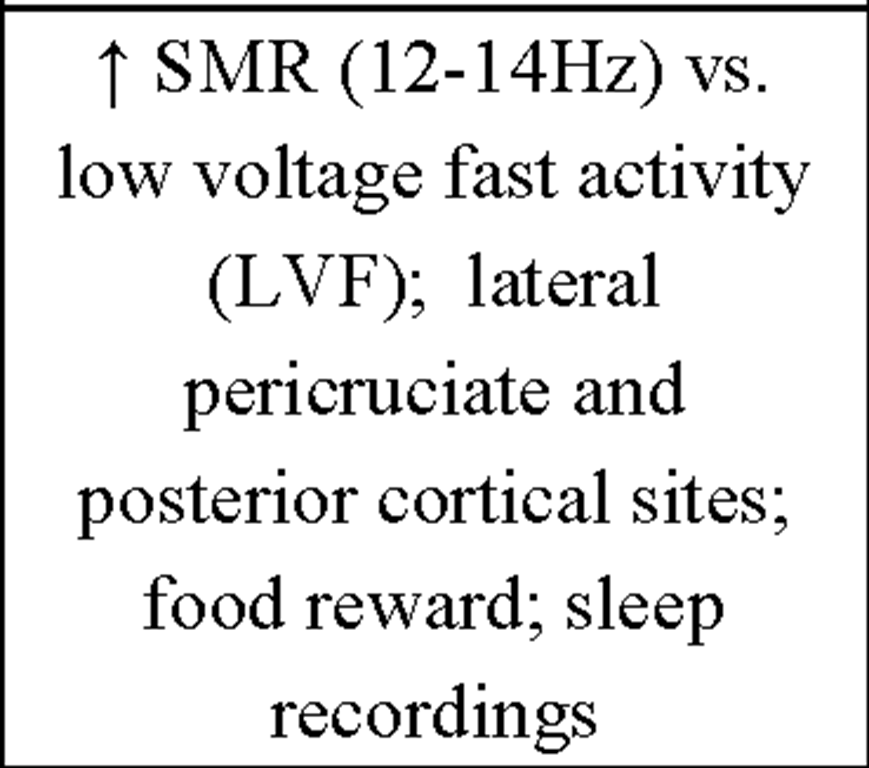
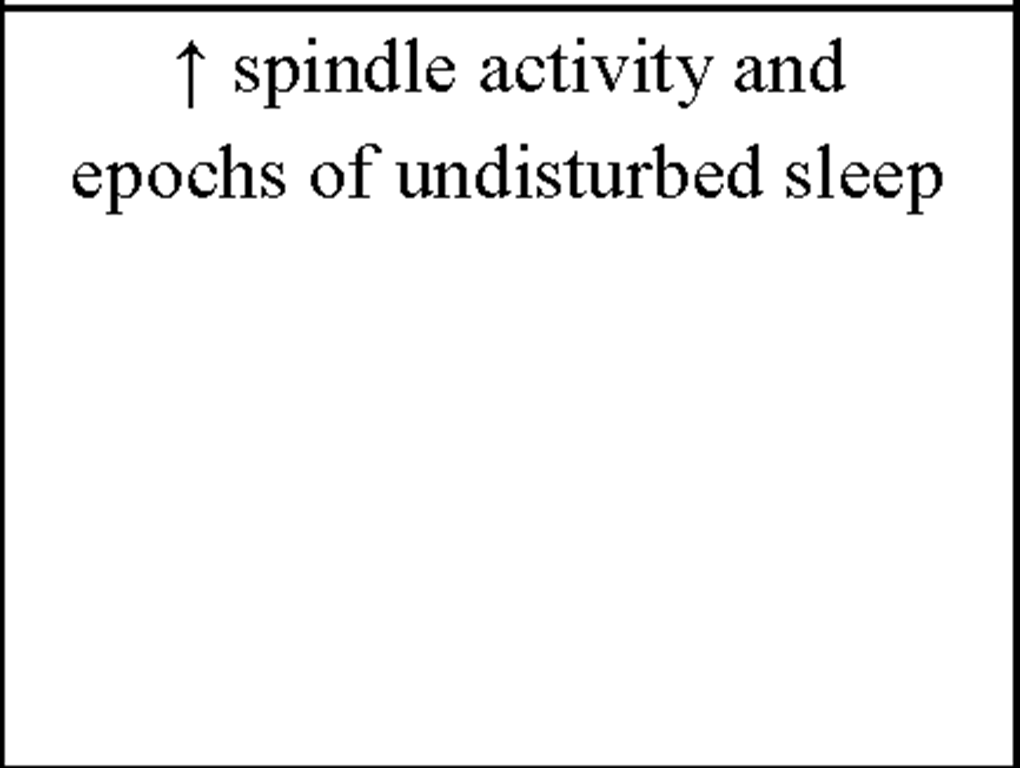
Of high interest for our approach are the early findings by Sterman et al. (1970) who could show that facilitation of sensorimotor rhythm (SMR) by IEC during wakefulness in cats (i) selectively enhances spindle activity during sleep and (ii) produces longer epochs of undisturbed sleep. EEG recordings over the sensorimotor cortex show a very distinctive oscillatory pattern in a frequency range between 12-15Hz termed SMR (Sterman & Wyrwicka, 1967; Chase & Harper, 1971; Howe & Sterman, 1972). These brain activities are also known as "rolandic mu rhythms" or "wicket rhythms" (Gastaut, 1952; Niedermeyer, 2005). SMR appears to be dominant during quiet but alert wakefulness (Roth, Sterman, & Clemente, 1967) and desynchronizes during planning, execution and also imagination of hand, finger, foot and tongue movements (Neuper, Wortz, & Pfurtscheller, 2006; Pfurtscheller, Brunner, & Schlogl, 2006). Active inhibition of motor behavior on the other hand results in SMR synchronization (Howe & Sterman, 1972). Furthermore this frequency range is known to be abundant during light NREM sleep, and is representing the classical sleep spindle band. First spindles emerge at sleep onset, have a waxing-and-waning appearance and are known to be generated in thalamocortical circuits (Steriade, 1999). Sterman and colleagues postulated that instrumental SMR conditioning can transfer into sleep, inducing a facilitation of spindle burst sleep and decreased sleep fragmentation (i.e., reduced waking and movements during NREM sleep) in normal adult cats. By instrumental conditioning the occurrence of SMR and the related suppression of movement could be induced in cats (Wyrwicka & Sterman, 1968). EEG was recorded from lateral pericruciate cortex (on both sides) and posterior cortical sites in eight cats. The animals were placed in a recording chamber equipped with an automatic feeding device. After adaptation to this chamber, three independent recordings of sleep were obtained as baseline. At least two sleep cycles (quiet sleep being interrupted eventually by periods of active sleep or spontaneous shifts back to the waking or hypnagogic state). Subsequently the eight cats were split in two groups of four each and both groups were trained to produce specific patterns of EEG activity recorded over sensorimotor cortex (either SMR or low voltage [<20^V], fast [18-30Hz] activity [LVF]) during daily sessions to receive food. One training session consisted of 60 reinforcements by food for producing the desired activity. In the SMR-condition a signal containing at least 0.5sec 12-14Hz activity at a voltage 100% above background level produced a reward. Most animals received maximum performance after 2-4 weeks of daily training. In a first test session cats were allowed to obtain unlimited reinforcement and remained in the chamber until several complete sleep cycles were recorded. In a second run training (SMR, LVF) was reversed for the two groups. A final sleep recording 1 month after the end of the second training block served as a follow-up. Results show that instrumental conditioning of SMR activity in the waking cat produce significant changes in spindle-burst activity (number and duration of spindle bursts) and sleep duration. An increase in spindle-burst activity during sleep following SMR was observed in both groups whereas spindle-burst activity in the follow-up 1 month later was enhanced only in the group receiving SMR-training in the second run, but was not sustained in the second group where intervening LVF conditioning was given. Additionally the mean duration of quiet-sleep epochs was significantly increased immediately after SMR conditioning, but not in the follow-up, supporting the findings by Roth et al. (1967) that phasic motor behavior suppression is related to SMR activity.
Moreover at those early times, 12-14Hz IEC has already been effectively used in treating patients suffering from psychophysiologic insomnia (Hauri, 1981; Hauri, Percy, Hellekson, Hartmann, & Russ, 1982). In Hauri et al. (1982) sixteen subjects suffering from psychophysiologic insomnia were randomly assigned to either an EMG / theta-feedback or EMG/SMR-feedback treatment group. At first subjects were evaluated for 3 nights in a sleep laboratory, including different psychological questionnaires, sleep logs and a psychiatric interview. Patients who satisfied criteria for psychophysiological insomnia defined by chronic, serious and relentless sleeping problems for at least 2 years; insomnia has been shown for at least 8 of the 14 nights reported by home sleep logs; >30min sleep latency or <85% sleep efficiency during the second and third laboratory night; no medical insomnia or serious psychiatric disorders) first received 6 frontalis EMG sessions to learn how to sit comfortably in an easy chair for at least half an hour and can relax at least to the degree that EMG artefacts on the EEG channel become rare. Once adequately relaxed, patients started with either Theta or SMR training sessions. All of them received 26 sessions of Theta or SMR training within 13 weeks. According to evaluations by home sleep logs both treatment-groups could benefit from IEC, whereas objective evaluations at the sleep laboratory revealed that tense and anxious insomniacs benefited only from theta but not from SMR training, while those who were relaxed but still could not sleep benefited only from SMR- but not from theta training. Therefore Hauri et al. (1982) could show that appropriate IEC has a long-lasting effect on insomnia, although patients have to be carefully selected, as the same type of IEC did not appear effective for all insomniacs.
A further approach of IEC patterns during wakefulness reaching translation into sleep EEG was presented by Amzica, Neckelmann and Steriade (1997). Cats were trained to generate fast (20-50Hz) oscillation bursts within the motor cortex (area 4) as well as the visual cortex (area 17). The training of each animal consisted of seven sessions of motor cortex conditioning, three sessions of extinction and seven sessions of visual motor cortex conditioning. Extinction sessions were used to abolish the local increase in generation of fast oscillation bursts and to reset the thalamocortical synchrony of fast oscillations to control values. During the training sessions every 10sec a light flash was delivered into the visual field of the cat (conditioned stimulus) – at least 200 stimuli per session. If there was a qualifying burst (conditioned response) produced within the next 2sec, the animal was rewarded by a jet of water 100ms later. The experimental paradigm was successful in conditioning an increase in generation of fast oscillation bursts within both locations. Furthermore, the increased burst-generation was associated with an enhanced synchrony of fast oscillations at different levels of the thalamocortical network. Most interestingly for the present investigation, the increased thalamocortical synchrony acquired during the conditioning sessions was also expressed during subsequent quiet waking, NREM sleep and REM sleep, indicating that the facilitation of the desired oscillation bursts through instrumental conditioning during wakefulness selectively enhances similar patterns during subsequent sleep (for details see Amzica et al., 1997).
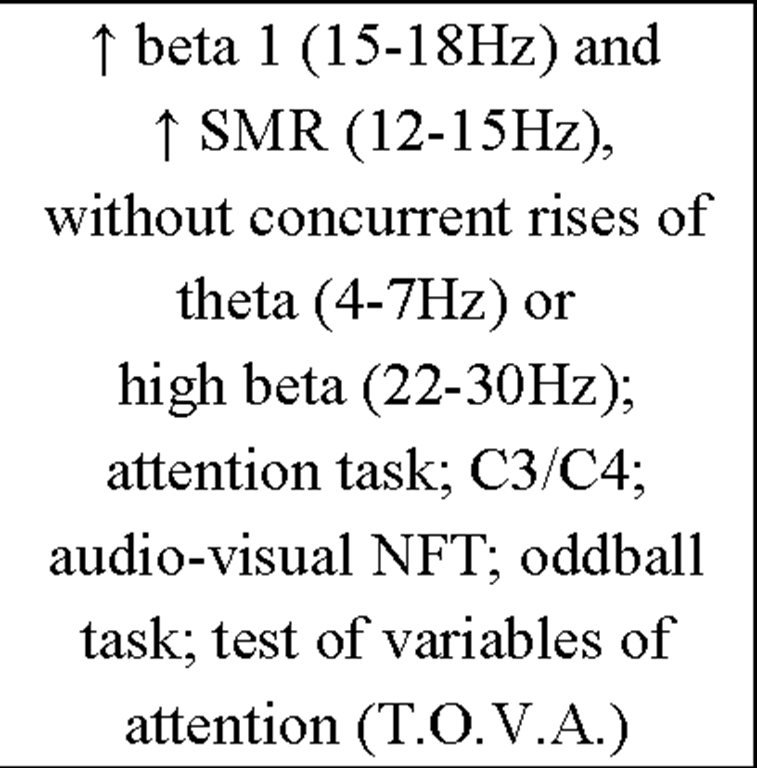
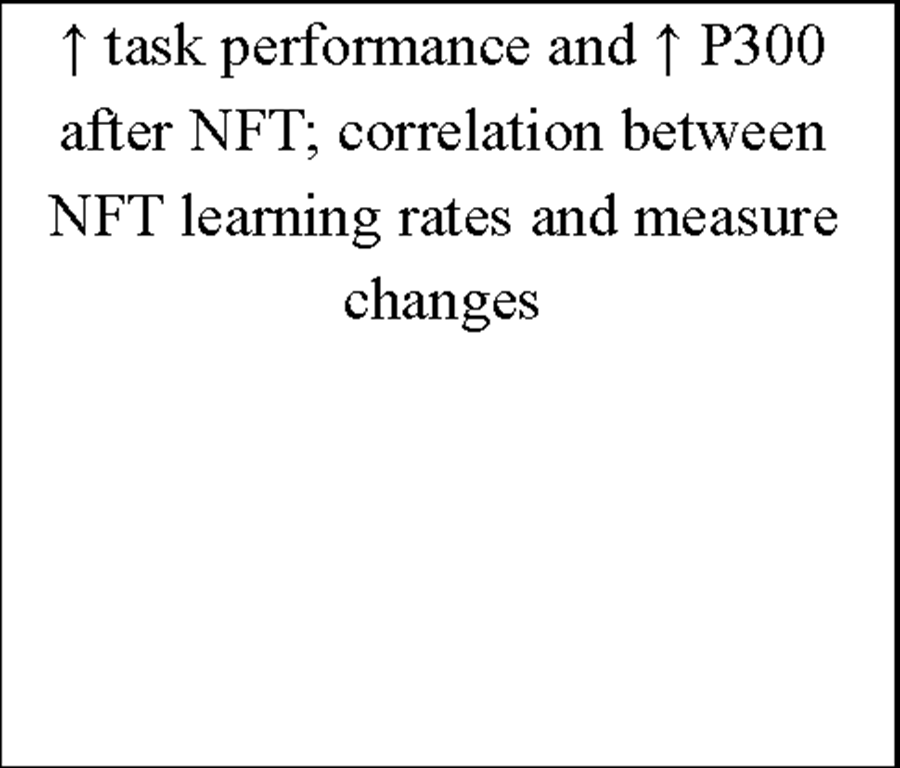
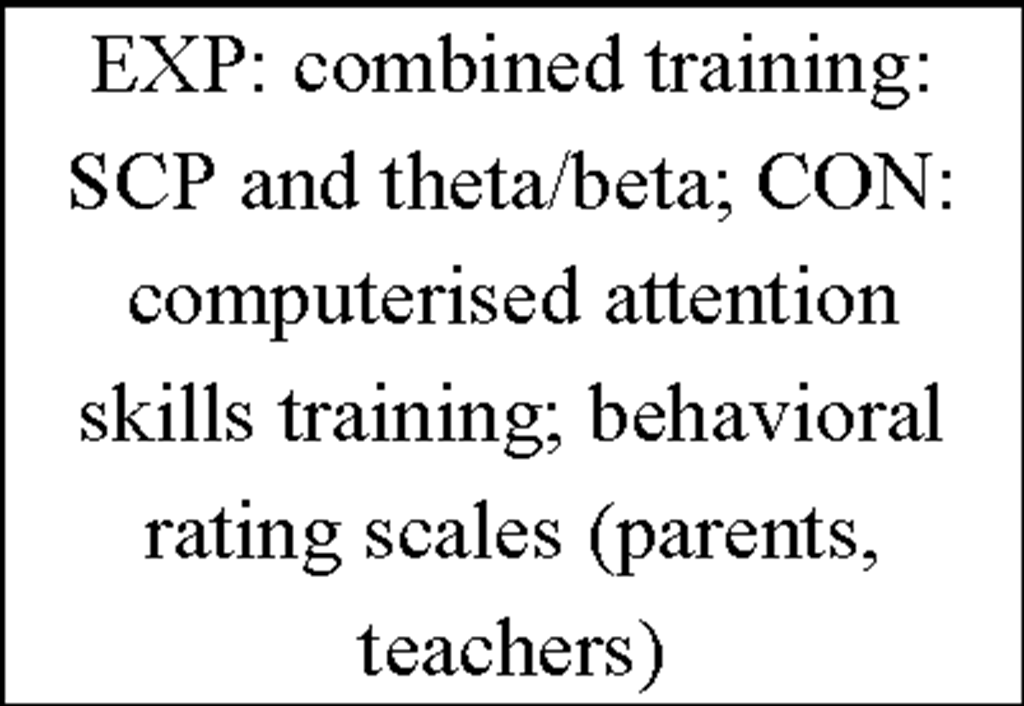
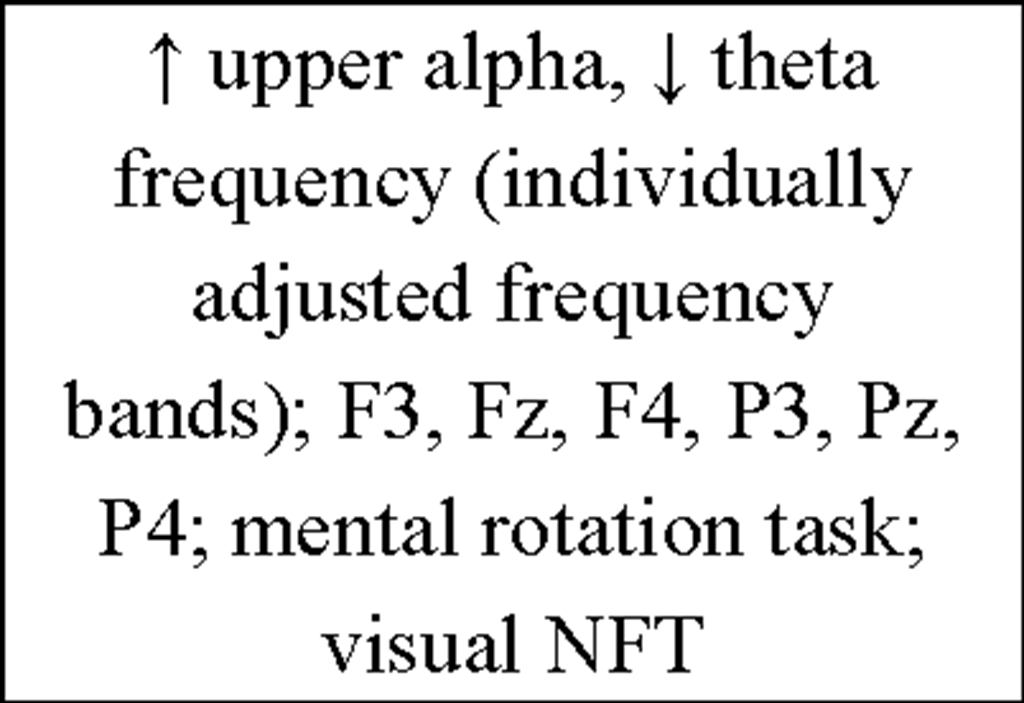
Additionally, more recent research focused on healthy individuals providing evidence that subjects who are able to gain control over different EEG parameters might even succeed in increasing performance levels in various tasks (for review see Gruzelier, Egner, & Vernon, 2006; Vernon, 2005). Those studies have pointed out that distinct IEC-protocols can be successfully used to improve attentional processing (Egner & Gruzelier, 2001, 2003; Egner, Strawson, & Gruzelier, 2002), increase accuracy in working memory tasks (Vernon et al., 2003) or improve performance in mental rotation (Hanslmayr, Sauseng, Doppelmayr, Schabus, & Klimesch, 2005).
Taken together there is a growing body of evidence suggesting that it is feasible to learn to regulate specific brain oscillations. Thereby it becomes possible to directly counteract the maladaptive brain activity which is associated with various disorders such as epilepsy, ADHD or sleep disorders. Unfortunately, much of the previous research concerning IEC has suffered from a lack of standardized measures of target symptoms, neglected the assessment of EEG changes and control groups or was conducted with insufficient sample sizes. Additional, well-controlled investigations are thus recommended before IEC can be considered a reliable non-pharmacological treatment for several disorders such as epilepsy, ADHD or even insomnia.
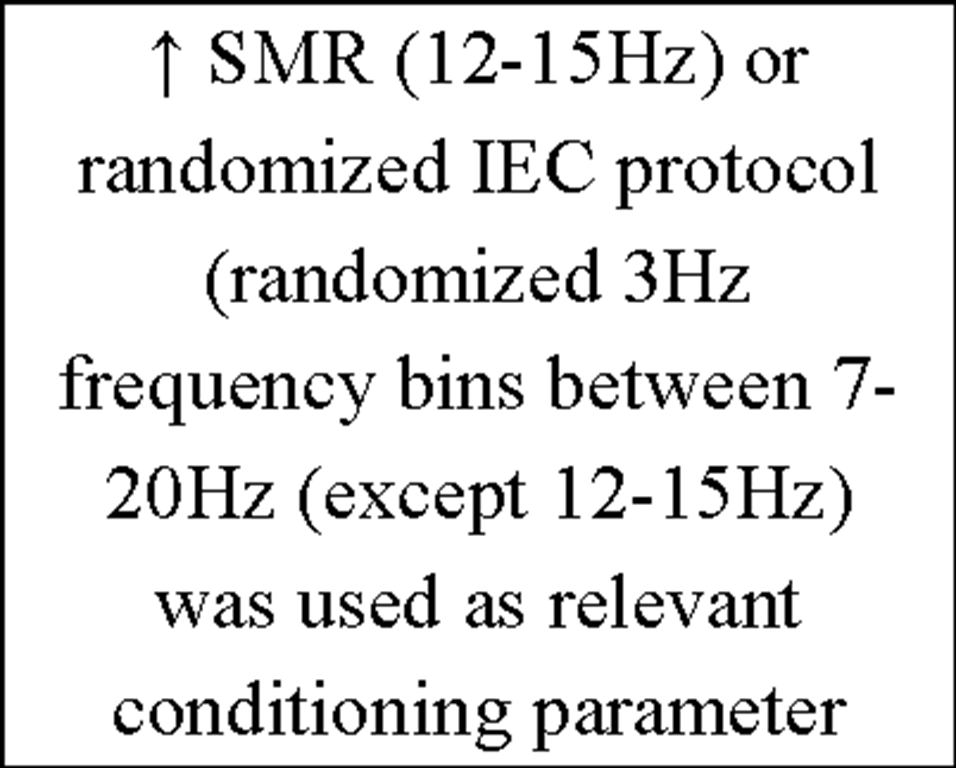
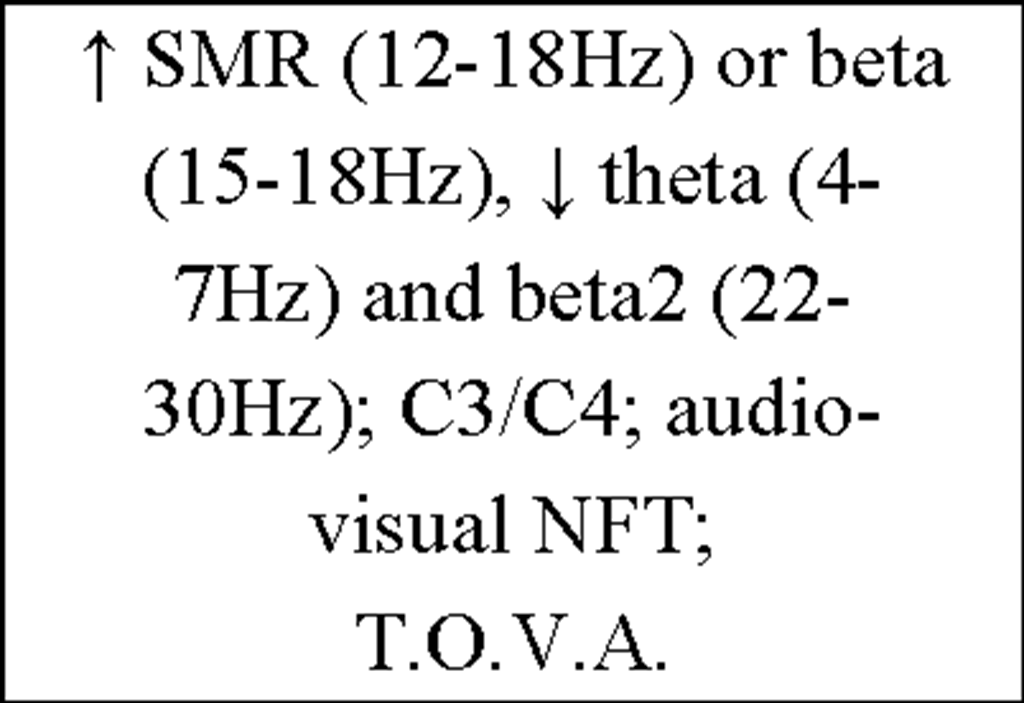
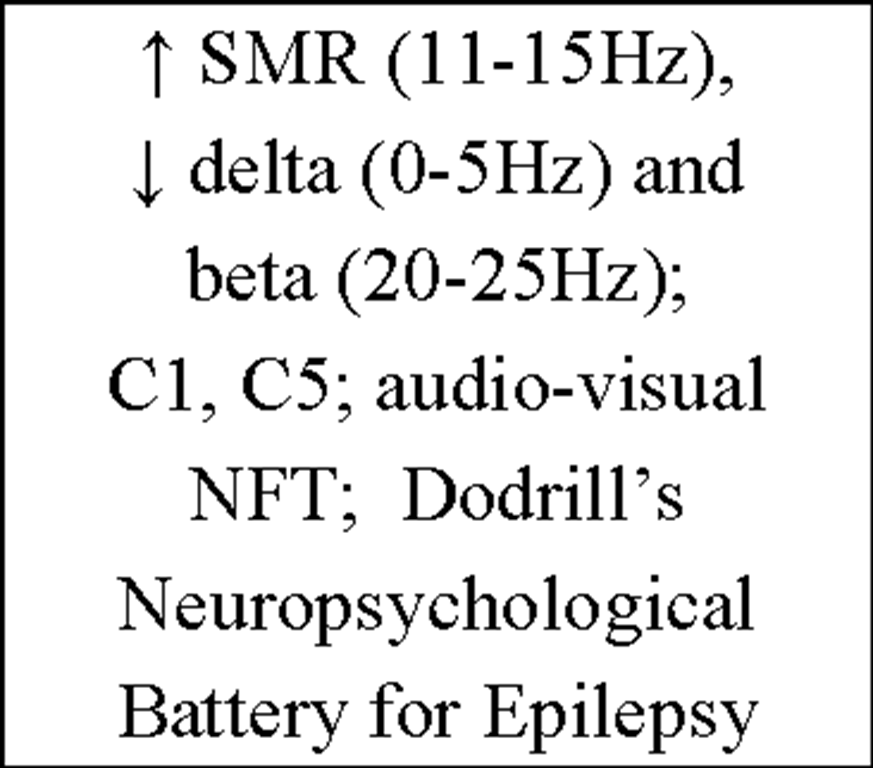
To provide an insight into the vast amount of studies concerning the issue "Instrumental EEG conditioning" an overview of the current IEC literature is presented in Table 4.
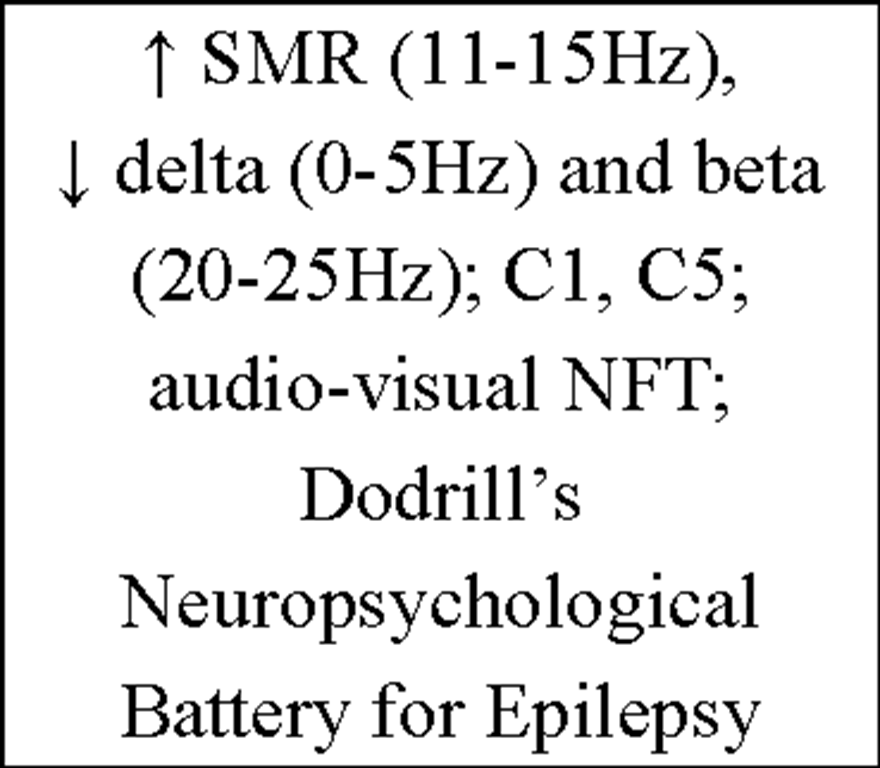
Table 4. Overview of current IEC literature.
|
Reference |
Subjects |
Feedback protocol |
Number and duration of sessions |
Results |
|
Berner et al. (2006) |
11 healthy subjects |
T 11.6-16Hz (sigma); Cz; sleep spindle activity + overnight memory performance change; neurofeedback-training (NFT) vs. pseudo-neurofeedback-training (PFT); visual NFT |
1 4 x 10 consecutive min |
T sigma power during subsequent sleep-stage 2-4 (NREM) |
|
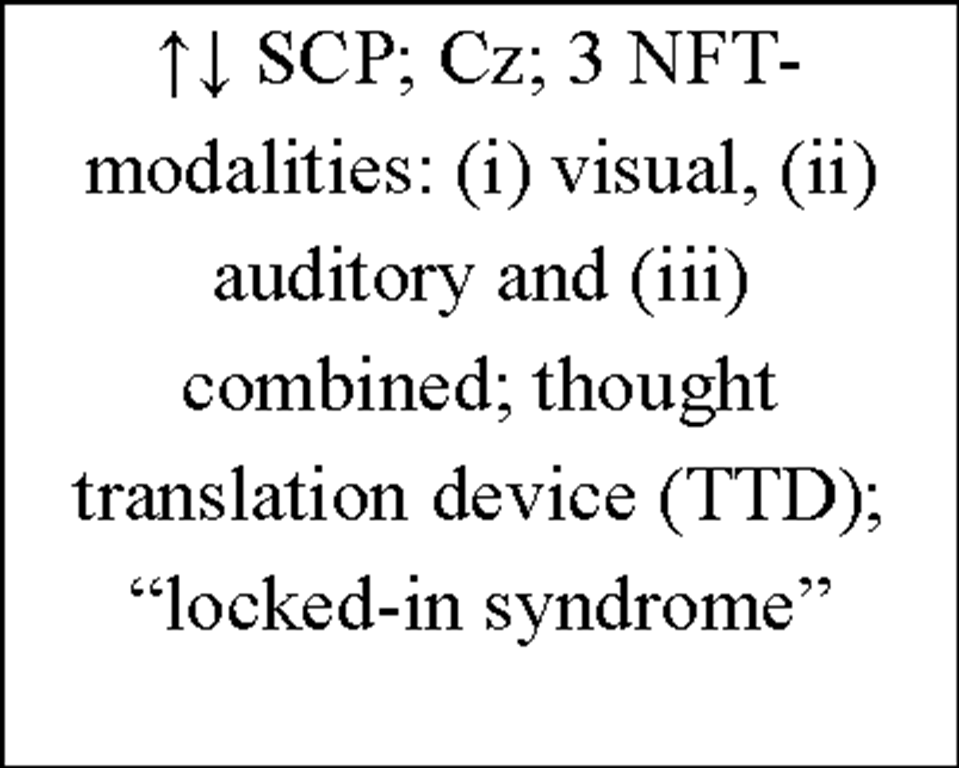
Birbaumer (1999) |
2 "locked-in" patients |
negative/positive slow cortical potentials (SCP); Cz; brain-interface; spelling device; imagery strategy |
288/327 6-12 per day 5-10 min |
both patients were better producing positive SCP; subjects were able to communicate by that kind of spelling device |
Table 4. Overview of current IEC literature.
Non-Pharmacological Alternatives for the Treatment of Insomnia
Table 4. Overview of current IEC literature.
Non-Pharmacological Alternatives for the Treatment of Insomnia
Table 4. Overview of current IEC literature.
Abbreviations:![]() = increase;
= increase;![]() = decrease; min = minutes; Hz = hertz; NREM = non rapid eye movement sleep; NFT = neurofeedback-training; PFT = pseudo-neurofeedback-training; SCP = slow cortical potentials; EXP = experimental group; SMR = sensorimotor rhythm; CON = control group; IVA = integrated visual and auditory continuous performance test; CPRS-R = Conner’s parent rating scale – revised; fMRI = functional magnetic resonance imaging; ADHD = attention-deficit hyperactivity disorder; T.O.V.A. = test of variables of attention; CBRS = Conner’s behavior rating scale; EMG = electromyography; EEG = electroencephalography; MMPI = Minnesota multiphasic personality inventory; TTD = thought translation device; FES = functional electrical stimulation; BCI = brain computer interface; LVF = low voltage fast activity; BOLD = blood oxygen level dependent; IQ = intelligence quotient; CPT = continuous performance task; CST = conceptual span task.
= decrease; min = minutes; Hz = hertz; NREM = non rapid eye movement sleep; NFT = neurofeedback-training; PFT = pseudo-neurofeedback-training; SCP = slow cortical potentials; EXP = experimental group; SMR = sensorimotor rhythm; CON = control group; IVA = integrated visual and auditory continuous performance test; CPRS-R = Conner’s parent rating scale – revised; fMRI = functional magnetic resonance imaging; ADHD = attention-deficit hyperactivity disorder; T.O.V.A. = test of variables of attention; CBRS = Conner’s behavior rating scale; EMG = electromyography; EEG = electroencephalography; MMPI = Minnesota multiphasic personality inventory; TTD = thought translation device; FES = functional electrical stimulation; BCI = brain computer interface; LVF = low voltage fast activity; BOLD = blood oxygen level dependent; IQ = intelligence quotient; CPT = continuous performance task; CST = conceptual span task.
![tmp115-64_thumb[1] tmp115-64_thumb[1]](http://what-when-how.com/wp-content/uploads/2012/04/tmp11564_thumb1_thumb.png)
![tmp115-65_thumb[1] tmp115-65_thumb[1]](http://what-when-how.com/wp-content/uploads/2012/04/tmp11565_thumb1_thumb.png)
![tmp115-66_thumb[1] tmp115-66_thumb[1]](http://what-when-how.com/wp-content/uploads/2012/04/tmp11566_thumb1_thumb.png)
![tmp115-67_thumb[1] tmp115-67_thumb[1]](http://what-when-how.com/wp-content/uploads/2012/04/tmp11567_thumb1_thumb.png)
![tmp115-68_thumb[1] tmp115-68_thumb[1]](http://what-when-how.com/wp-content/uploads/2012/04/tmp11568_thumb1_thumb.png)
![tmp115-69_thumb[1] tmp115-69_thumb[1]](http://what-when-how.com/wp-content/uploads/2012/04/tmp11569_thumb1_thumb.png)
![tmp115-70_thumb[1] tmp115-70_thumb[1]](http://what-when-how.com/wp-content/uploads/2012/04/tmp11570_thumb1_thumb.png)
![tmp115-71_thumb[1] tmp115-71_thumb[1]](http://what-when-how.com/wp-content/uploads/2012/04/tmp11571_thumb1_thumb.png)