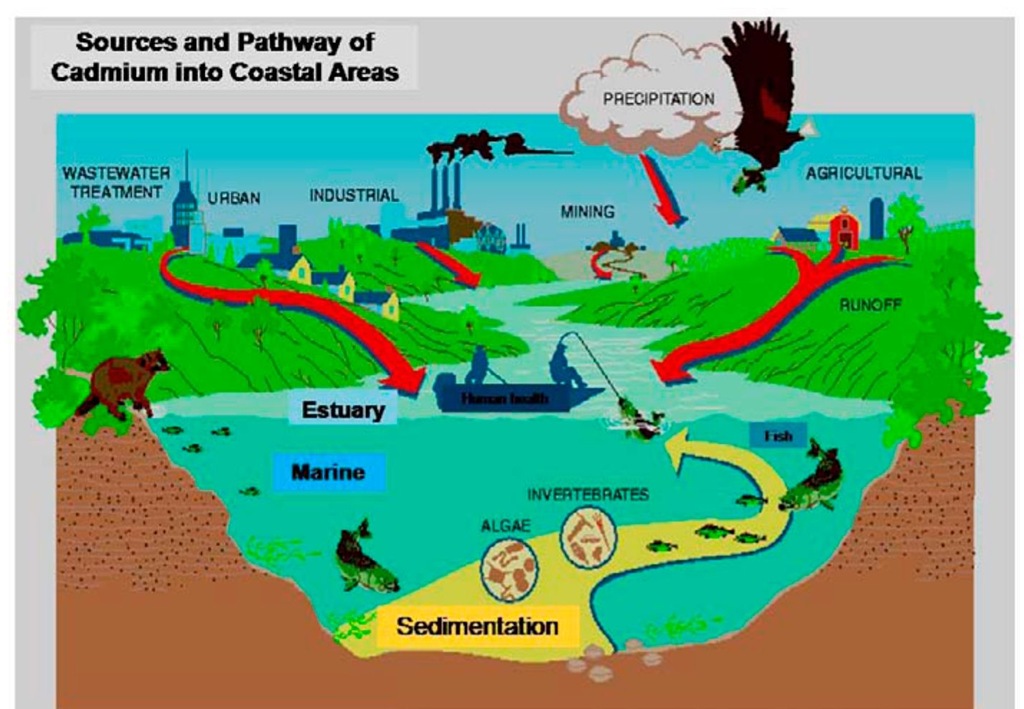
As already noted, cadmium may be present in the environment both from natural and anthropogenic sources. It occurs mainly as a component of minerals in the earth’s crust at an average concentration of 0.18 mg/kg and usually ranges from approximately 0.01 to 1.8 mg/kg in soils. In natural freshwaters, cadmium can occur at concentrations below 0.1 ^g Cd/L, but in environments impacted by human activities, concentrations can be several micrograms per liter or greater (USEPA, 2001). The various sources of cadmium to the environment are shown in Figure 1. The emission ratio of anthropogenic to natural cadmium can be as high as 7:1.
Background concentrations of cadmium in waters range from <2 to 16 ng/L for pristine freshwaters where the cadmium cation predominates, and <5 to 100 ng/L in saline waters where cadmium is mainly complexed by chloride. Open seawater cadmium varies between 0.02 and 0.1 ^g/L. The cadmium cation is the most bioavailable chemical form. Other factors affecting cadmium bioavailability in water include salinity, pH, dissolved organic carbon, and water hardness (calcium and magnesium). In sediments, humic material and acid volatile sulfides (FeS) will be important controls on cadmium bioavaiability. Other ions that can affect cadmium uptake or toxicity include manganese, zinc, and selenium.
In recent years, much concern has been expressed about the fate (transport, distribution, and transformation) of this potentially toxic metal in aquatic ecosystems.
Figure 1. Different sources and pathway of cadmium into the aquatic environment.
Natural Occurrences
Cadmium occurs in nature as a natural component of rocks, sediments, soils and dusts, air, water, and plant and animal tissues, where it appears to cause no harm either to human beings or to the environment. Its geochemical behavior is similar to that of zinc because of the similar electron structures and ionization potentials of the two elements.
The cadmium concentration found in rocks varies widely, ranging from 0.1 to 1 g/kg in soils, and to 100 g/kg in phosphatic rock. Cadmium forms an oxide and carbonate under natural conditions and is a constituent of both primary zinc minerals such as sphalerite and secondary minerals such as smithsonite. Because of its high mobility in the environment, large amounts of cadmium reach the water cycle, leading to extensive particle formation and subsequent sedimentation. Remobilization from sediments also occurs, enhanced by numerous chemical and biological factors, leading to the widespread dispersion of cadmium into ground water. Cadmium in marine sediments, range from 0.1 to 1.0 mg/kg in the Atlantic and Pacific oceans (Thornton, 1992).
Soils tend to reflect the chemical composition of the parent materials from which they were derived. The average cadmium content of surface soils ranges from 0.07 to 1.1 mg/kg in many parts of the world and all values over 0.5 mg/kg reflect anthropogenic inputs (Thornton, 1992).
Cadmium enters the atmosphere through natural processes such as weathering and volcanic emissions and is then deposited by precipitation into water bodies. In unpolluted and uninhabited areas, cadmium concentrations in the air are less than 1 ng/m3.
Cadmium tends to accumulate in the leaves than in seed or root crops of plants that are grown on cadmium-containing-soils. Cadmium concentrations vary widely but generally range from less than 5 mg/kg to 10 mg/kg in most mammals. In oysters, mussels, amphipods, and squid, cadmium accumulates to concentrations of more than 1 mg/kg, whereas the concentrations in fish tend to be lower. Such low concentrations are due to cadmium sequestration by phytoplankton and/or the feeding behavior of invertebrate organisms (Lee et al., 2000). In humans, the main route of exposure to cadmium under normal conditions occurs through dietary ingestion and inhalation of tobacco smoke.
Anthropogenic Sources
World production and consumption of cadmium has continued to rise in most industrialized countries in recent years, principally as a by-product in zinc refining.
The end uses of cadmium in different industrial processes include production of television tube phosphors, preparation of special alloys and solders, metal plating, nuclear reactor shields and rods, pigments in yellow or brown paints (for coloring plastics, glass, and enamels), stabilizers for processing PVC polymers, nickel-cadmium rechargeable batteries, and electronic waste. Cadmium coating are mainly applied via electroplating or dipping to another metal as a thin film for protection against corrosion. Seldom is it possible to recover the metal economically. The use of cadmium in alkaline rechargeable batteries has potential environmental hazards in view of the amounts of nickel and cadmium involved. These uses lead to the flow of cadmium into the environment in the form of atmospheric emissions, liquid effluents and wastewaters, as well as sludges and solid wastes.
Chemical fertilizers of the super-phosphate group can contaminate the soil because they contain from 0.05 to 170 mg Cd/Kg. In addition to the above-mentioned sources, mining also provides at least two indirect sources of environmental cadmium. Firstly, the products of fossil fuel combustion in thermal power stations are the source of 50% of the atmospheric cadmium emissions. Secondly, cadmium that is associated with zinc ores does not remain contained within the localized mining region but rather seeps into local soils and drainage waters (Pinot et al., 2000). So anthropogenic sources, including smelter emissions and the application of fertilizers and sewage sludge to land may lead to contamination of both soils and crops. Besides incidental exposure through anthropogenic sources, tobacco smoke represents a major source of consistent and self-induced exposure to cadmium.
The majority people of the world live in town and cities and are subjected to exposure in the urban environment. The major anthropogenic sources of cadmium in the ambient air are non-ferrous metal smelters and uses of coal for combustion. Cadmium concentrations in the surrounding air depend on the population and urbanization of the region. Ambient air cadmium concentrations have been generally estimated as extremely low and may range from 0.1 to 5.0 ng/m3 in rural areas, from 2.0 to 15.0 ng/m3 in urban areas, and from 15.0 to 150 ng/m3 in urban and industrialized areas, particularly near metal refining and processing plants (OECD 1994). In urban areas, the cadmium concentrations may be higher due to various urban, transportation and combustion activities.
In house dust, cadmium is approximately six times greater than the concentration in soil, which presumably originates from cadmium-containing materials such as abraded plastics and possibly paints, presenting a possible risk to young children, who may accidentally ingest it. In addition, cadmium may be introduced by urban and motorway dusts, all of which are especially important considering the increasing development in populated, urban environments (Culbard et al., 1988).
In summary, the main anthropogenic sources of cadmium in water relate to ore mining (including mining wastes and mine waters), metallurgical industries, industrial use, municipal sewage effluents, cadmium-contaminated phosphorus-containing fertilizers, the disposal and application of sewage sludges on land, and to the contaminated agricultural soils. Furthermore some researchers have shown that most cadmium additions to water or land are from atmospheric deposition (Nriagu and Pacyna, 1988). So these sources, together with atmospheric precipitation, are thought to constitute the critical pathway for the contamination of organisms and of humans through the food chain.
Status of Cadmium Pollution in Estuarine and Coastal Areas
Anthropogenic and airborne sources of cadmium entering the seas and oceans directly from atmospheric deposition mainly in particulate forms and, to a lesser extent, dissolved in rain and snow and indirect via river runoff.
Rivers are subjected to cadmium inputs from raw or treated sewage. River sediments generally reflect the neighbouring soils and mineral workings. High cadmium levels are invariably accompanied by high levels of other trace metals. As a result of these inputs, there are enhanced levels of cadmium in near-shore sediments and coastal sea-water. For example, the River Rhine has a range of 1 to 10 g Cd/L. The general range for clean rivers and lakes is about 0.1 to 1.2 ^g Cd/L, but 1 to 36 g Cd/L in polluted industrial rivers in the United Kingdom, the USA, and in Europe. Values of 1 to 9 ^g Cd/L were found for the Jintsu river in Japan which flows through the area where Itai-itai disease occurred and 30 ^g Cd/L was found in the drainage streams of a nearby ore mine. In the United Kingdom, 3 to 95 ^g Cd/L was found in streams in North Wales, while 5 to 20 g Cd/L was found in areas in Cornwall affected by ore mining.
The transport of cadmium from freshwaters to the sea occurs either in particulate or soluble forms. The specific form depends on the state of the river, its mineralization and its sources of pollution, as well as on unidentified local factors. So quantification is difficult and studies on the transfer of the different forms of cadmium from freshwater to the sea are inconclusive. Certain estuaries are conservative in most trace metals. The daily inputs to inshore waters of the North Sea from the Humber estuary have been assessed as 37.15 kg Cd/d. The relative proportions and magnitudes of these inputs should be of general interest to other fairly heavily urbanized and industrialized estuary areas (UNEP, 1985 and literature cited by this report).
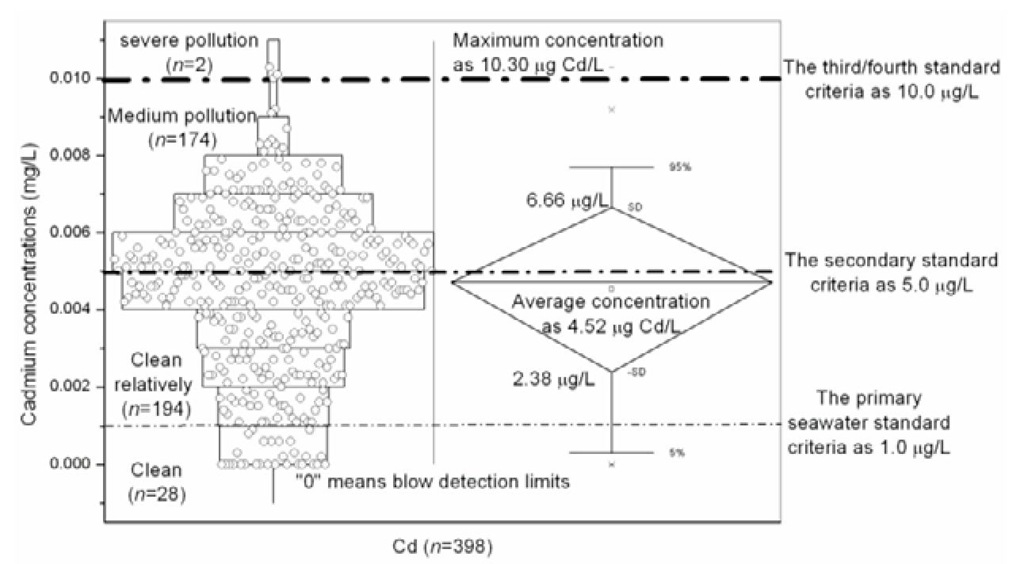
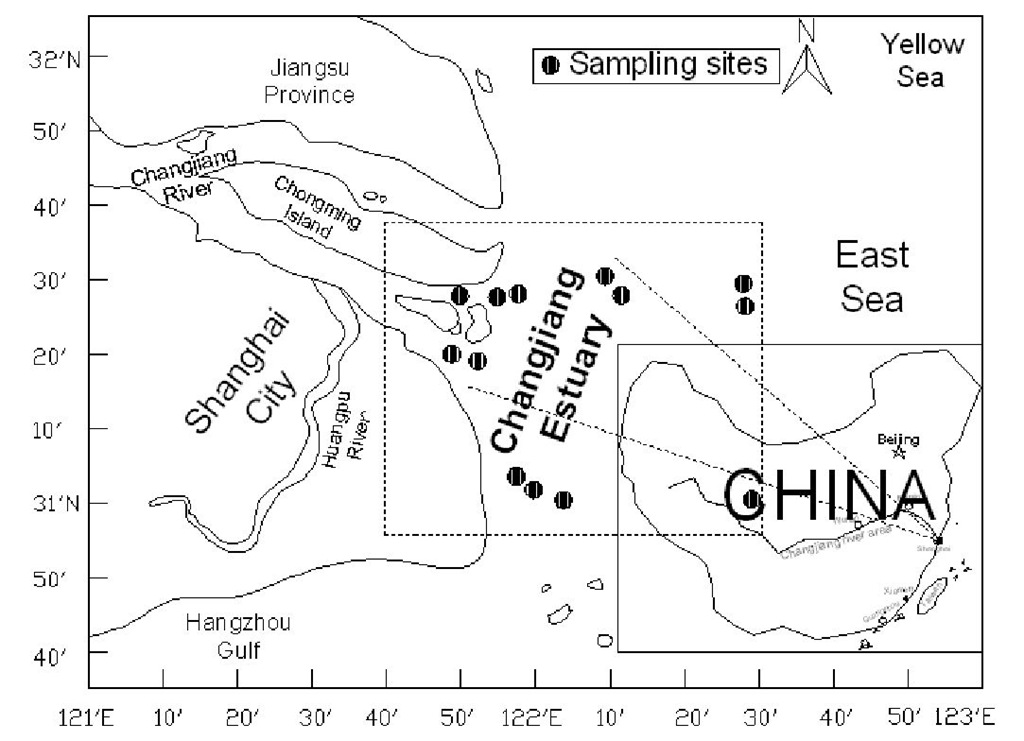
In China, heavy metals are continuing to be introduced to estuarine and coastal environment through rivers, runoff, and land-based point sources with the rapid industrialization and economic development. The Changjiang estuary is the largest in the western Pacific Ocean and the fifth largest in the world, and is located near Shanghai (the largest city of China). It receives a large amount of industrial discharges, urban waste disposals, and agricultural and domestic sewage effluent from the Changjiang delta area (the most economically developed area in China) and Changjiang River water. Figure 2 shows cadmium concentrations in various estuarine water from 13 sampling sites of Changjiang estuary, all sampling sites of which are located in latitude 30°54′N – 31°38′N, longitude 121°40′E – 122°30′E (see Figure 3). Depth profiles showed that the average cadmium concentrations of water column were 4.40 ± 2.20^g Cd/L (n=122, surface layer), 4.48 ± 2.05 ^g Cd/L (n=118, middle layer), 5.02 ± 2.13^g Cd/L (n=118, bottom layer), respectively.
Figure 2. Distribution of cadmium concentration measured in Changjiang estuary and comparison with seawater standard criteria.
Figure 3. Locations of the sampling sites and schematic map of the Changjiang estuary, China.
Cadmium in aquatic ecosystems tends to accumulate in sediments, acting as a source for benthic biota and possibly for re-entering the water column. Measured cadmium concentrations of surface sediments varied from 0.11 to 1.11 mg/kg in Xiamen Bay (sampling sites shown in Figure 4). Taken together, these coastal and estuarine monitoring data suggest the presence of cadmium contamination and polluted situations in China* (Wang et al., 2009a).
Figure 4. Locations of the sampling sites and schematic map of the Xiamen West Sea area, China.
The high cadmium exposures in estuarine and coastal areas of China are most likely related to human activities, such as industrial and municipal effluents, landfill leaching, non-point source runoff, and atmospheric deposition, although a direct link cannot be established with the available data.