ABSTRACT
Shape-Memory-Polymers (SMP) is smart materials have the ability to memorize original shape; and can be used as sensors, transplants, and structural materials in engineering applications. Despite its practical importance, limited work is available on its degradation behavior. This study has been carried out to evaluate degradation behavior of Veriflex-SMP on exposures to water and diesel fuels separately. It is found that "glass transition temperature, Tg" decreases due to immersion in liquids; and immersion facilitates acceleration of breakage of the existing bonds and the formation of new bonds, thereby increasing the mobility of polymeric chains; and the immersion medium effectively plasticize SMPs by reducing storage modulus and decreases structural integrity of SMP. Upon exposure to diesel fuel for several weeks, "Tg" goes below room temperature; and SMP goes back to its original shape without the need of the application of external energy. Stress relaxation tests shows that stress decay is found to be much faster and to a much lower value in degraded samples and increases with increase of immersion time. From Fourier Transform Infrared tests formation of hydrogen bonding between SMP and the solvents in which it is immersed, is the main reason for SMP degradation; although hydrogen has a minor effect on SMP structure but has an obvious influence on glass transition temperature, Tg.
Keywords: SMP (Shape-Memory Polymer), degradation behavior, glass transition temperature, bond formation, polymeric chains, stress relaxation, Fourier Transform Infrared test
INTRODUCTION
Shape memory polymer (SMP) is a type of shape memory material, which has been investigated in this research. The SMP is a smart material whose property can be altered in a controlled way and has the capability of recovering large deformation. Smart material has the ability of sensing, processing, actuating, self-diagnosing, and self-recovering. They can sense the change in environment and act accordingly to minimize the action of the changes in environment; similar to a living being that can sense, make decisions, and take actions. Their physical and chemical properties are sensitive to the change in environment such as pressure, temperature, electric field, pH level, magnetic field, etc. They utilize their own properties and functions to achieve smart action. This material has the ability to return from its temporary deformed shape to a permanent shape upon the application of external stimulus such as heat, i.e., it remembers its original shape. They are stimuli-responsive materials; for example, a temperature responsive shape memory material is the one that undergoes a structural change upon reaching a certain temperature, called the "transition temperature", where the polymer goes from a brittle or glassy state to a flexible or rubbery state. The main advantage of shape memory polymer is the ability of recovering a large amount of strain (usually >400%) in comparison to shape memory alloys (only up to 15%) and shape memory ceramics (2 – 3%) [Hu, 2007]. 1.1 Structure of Shape Memory Polymer
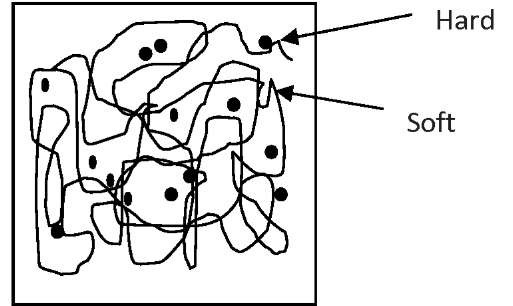
Generally, SMPs are phase-segregated linear block copolymers having a "hard segment" and a "soft segment" in terms of molecular structure (Fig. 1). Hard segments (or crystalline segments) are responsible for maintaining the permanent or original shape through intra or inter polymeric chain attractions such as hydrogen bonding or dipole-dipole interaction together with the physical or chemical cross-linking. Whereas, the soft segments (or amorphous segments) are responsible to freely absorb external stress by unfolding and extending their molecular chains.
Fig.1: Network structure of SMP showing hard and soft segments
(i) Glass Transition Temperature, (Tg) is above the transition temperature of the soft or amorphous segment (Tgs) but below the transition temperature of the hard or crystalline segment (Tgh). At this transition temperature
the crystalline segment will not be affected by the temperature whereas the amorphous segment will be melted and will absorb the deformation. In the case of amorphous based shape memory polymer, when the temperature of the SMP will go below the glass transition temperature, the amorphous segments will begin to rearrange themselves but due to the presence of crystalline segment they will not be able to go back to the original shape and consequently, the deformation will be locked in. The shape memory effect not only depends on the specific material property of a single polymer but it results from the combination of the polymer structure and morphology together with the applied processing and programming technology [Hu, 2007]. It is known that the macroscopic properties of polymer can be controlled by specific variation of molecular structure. This can be used to tailor the specific properties of shape memory polymer by slight variation in molecular structure [Lendlien & Kelch, 2002].
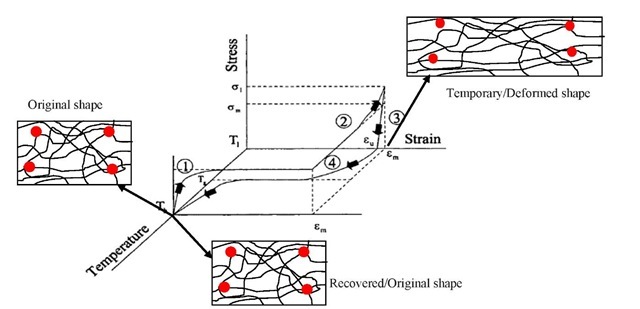
(iii) Thermo-mechanical Programming: In order to use the shape memory functionality of SMP, we need thermo-mechanical programming (see Fig. 2). For programming, the shape memory polymer is heated above the transition temperature, which can be glass transition or melting transition. At this temperature the shape memory polymer is in rubbery state and it can easily be deformed i.e. it can accommodate deformation. Now at this stage, a force is applied on SMP, which induces strain in the polymer (process-1). After deforming, it is cooled below the transition temperature, at which the switching segment becomes inactive and the polymer is in frozen or glassy state (process-2). After that, the applied load has been removed. On the removal of load, the polymer recovers some of its deformation due to elasticity behavior; but most of the deformation has been locked- in and the polymer remains in the deformed state because of the decrease in temperature from above the transition to below the transition temperature (process-3). The resulting deformation creates a residual stress/strain which remains in the polymer until the recovery. Now at this stage if we heat the polymer above its glass transition temperature and it will go back to its original shape (process-4) [Lendlien & Behl, 2007].
Polymer Properties: (i) Visco-elastic Properties: Polymers are neither perfectly elastic nor perfectly viscous material, but they show deviations from idealistic behavior; such as: (i) The strain (in a solid) or the rate of strain (in a liquid) is not directly proportional to stress. In fact, there exists no clear relationship; (ii) Stress may depend on both the strain and the rate of strain as well as higher time derivative of strain. Because of these deviations, polymer posses both solid as well as liquid like characteristics and are termed as visco-elastic material their relationship between stress and strain depends on time.
Fig. 2: Thermo-mechanical cycle of a thermally induced shape memory polymer
The most important visco-elastic properties of polymer are: (a) Storage Modulus (E’), (b) Loss Modulus (E"), Tan delta (Tan . ), (d) Complex Modulus (E*): and (e) Transition Temperature, Tg- As the temperature of polymer increases most of the polymer chains begin to relax and move. As a result, free volume of polymer increases significantly which is accompanied by drastic change in property. The glass transition temperature is the temperature at which the polymer undergoes a drastic change in the property such as mechanical, electrical, thermal, or other electrical property. The glass transition temperature is dependent on the degree of crystallinity of the polymer. In this study, the glass transition temperature has been determined by DMA (Dynamic Mechanical Analyzer). In order to characterize the shape memory properties of polymer a set of parameters such as (i) Strain Recovery Rate, (ii) Shape Fixity Rate, (iii) Recovery Rate, and (iv) Recovery Stress are determined.
1.2 Research Objectives: Shape memory polymers exhibit a unique combination of low density, higher recoverable strain, and shape memory behavior that gives them a wide range of potential applications. Earlier works have considered the effect of limited environmental factors on shape memory polymers, and focused on polyurethane based shape memory polymers. This study is to provide an additional insight of the effect of environmental factors on SMP such as the thermo-mechanical cycle, water absorption, moisture, heat treatment, and dynamic loading. This study examines the degradation behavior of Veriflex-SMP, (from Corner Stone Research Group) due to prolonged immersion in water and diesel fuel separately.
BACKGROUND RESEARCH
The shape memory effect was first reported by Chang and Read in 1932 [Chang & Read, 1951]. Thermally responsive shape memory materials are the most notable one, in which the recovery takes place upon heating it above the transition temperature. According to Sakurai et al. [Sakurai & Takahashi, 1989] the first SMP, developed by the French CDF Chimie company and commercialized by Nippon Zeon Co. in Japan, was polynorborene based SMP with the glass transition temperature, Tg ranging from 35°C to 40°C, which was suitable for developing apparel for textiles. However, the molecular weight of this polymer is very high which makes it difficult for processing. Later Kurray company in Japan developed a transpolyisoprene based SMP with a Tg of 67°C in 1987. Asahi Company also introduced a styrene-butadiene based SMP with the Tg ranging from 60°C to 90°C [Shizuo & Nobuo, 1991]. The high transition temperature, Tg of this two types of SMP serves them as a good candidate for high temperature application but limit their usefulness in textile apparel. Finally, Mitsubishi Heavy Industry developed a polyurethane based thermoplastic polymers (SMPU) with transition temperature, Tg ranging from -30°C to +65°C. Apart from that, the processibility of SMPU is also easy [Hayashi & Shirai, 1988]. Since the discovery, SMP have drawn increasing attention because of its high recoverable ratio, low cost, low weight, and wide range of mechanical properties as compared to SMAs or ceramics [Liu et al., 2007]. To fully utilize the shape memory functionality of shape memory polymer and explore new area for application of shape memory polymer demands thorough characterization of SMP. A comprehensive description of the recent development of SMP has been reported by Nahid (2010). In order to explore possible new applications of SMP, it is necessary to determine the material resistance against various environmental factors. Huang et al., 2005 examined the effect of moisture on the Tg and shape memory property of SMP. Yang et al., 2006 found that the Tg of the polymer decreases and ultimately the SMP losses its shape fixing capability if it is left in air at room temperature for several days.
EXPERIMENTAL METHODS
A brief description of experimental methods is given in this section. Materials: In general, the SMP can be manufactured by mixing one reagent containing two active amino hydrogen or two active phenolic hydrogen with at least one multifunctional cross-linking reagent which contains at least three or more active amino or phenolic-hydrogen. The mixture is further mixed with at least one difunctional epoxide. In this case the SMP is the product of reaction between (a) styrene, (b) a vinyl compound other than styrene, (c) a cross linking agent, and (d) an initiator. By varying the composition of each component, the Tg of SMP can be tailored to the required application.
3.1 Properties of Material: A few important properties of styrene based Veriflex- SMP supplied by manufacturer are: Ultimate Tensile strength (23 MPa), Flexural strength (37.1 MPa), Compressive strength (32.4 MPa), Thermal conductivity (0.17 W/(m-°K), Tensile elongation to break (3.9%), Glass transition temperature (143°F ( 62°C)), Thermal conductivity (0.17 W/( m-°K), Material density (0.92 g / cm3).
Experimental Program:
The general degradation process of polymer is of two types: (i) Physical, and (ii) Chemical. To investigate the effect of degradation we used only two different types of solvent: water and diesel fuel: (i) Water: In this research distilled water at Room temperature has been used. Moisture varies from 10% to 100% depending on the weather condition. According to the knowledge of the authors, no research has been carried out on Styrene based SMP. (ii) Diesel fuel: Automotive diesel fuel has been used as another solvent, which is a strong solvent. For designing new application of SMP the effect of diesel fuel on its property must be considered as exposures to diesel fuel is quite common. Four experiments were performed: Thermo-mechanical cycle, Dynamic mechanical thermal analysis, Stress relaxation test, Fourier transform infrared test.
Sample Preparation: Commercial styrene based SMP samples were received as thin sheet. Then the samples of desired dimensions were cut according to the test requirements. After the cut, the samples have been polished using 1200 grit sandpaper to remove surface roughness.
Equipments: In order to measure the effect of degradation due to water and diesel fuel several tests such as: shape memory capability, recovery force, visco-elastic properties, and change in molecular vibration tests several equipments are used: Dynamic Mechanical Analyzer (DMA -2980) is an analytical instrument used to measure the physical properties, mainly visco-elastic properties of many different types of material. The tensile clamps are used which consists of two clamps: movable and fixed clamp. The movable upper clamp applies sinusoidal force on the sample with the help of a variable speed motor. Due to the application of applied force, the sample undergoes sinusoidal deformation, which is not in the phase of applied force because of visco-elasticity property.
Rheometric Scientific Analyzer, RSA-III (Fig. 3 -shown left) with the tensile clamp shown in figure has been used in this study.
It uses a servo linear actuator to impose an oscillatory deformation or strain, mechanically upon the material to measure the dynamic mechanical properties.
Fourier Transform Infrared (FTIR) Spectroscopy:
To determine the root cause of a degradation process in a polymer it is necessary to identify chemical properties of an unknown substance or contaminants. The identification becomes more challenging when the contaminant is organic in nature because of the presence of large number of organic compounds. The basic principle of FTIR lies on the absorption of infrared photons, which excite vibrations of molecular bonds. The amplitude of vibrations or a rotation of chemical bonds increases at specific frequencies corresponding to discrete energy levels due to the interaction of infrared light with the matter. This frequency is related to the shape of the molecular potential energy surfaces, the masses of the atoms, and the vibronic coupling. In this way, the presence of a functional group in a sample can be identified by the correlation of the bond wave number position with chemical structure.
Dynamic Mechanical Thermal Analysis (DMTA) Tests: To investigate the change in dynamic mechanical properties of virgin SMP samples and the one degraded by water and another one by diesel fuel as a function of time, DMTA tests (Fig. 4) have been performed on RSA- III, in strain-controlled dynamic temperature ramp mode at a constant frequency of 1.0 Hz in the temperature range of 30°C to 100 °C at a heating rate of 3 °C/min. The tensile clamp has been used and the gaps between the clamps are 15 mm. (Fig. 4: Experimental Setup-shown left). Recovery Force Measurement: In order to measure the recovery force and time, a two- stage process was developed. In the first stage, the temperature of the sample has been raised to above the glass transition temperature to accommodate deformation. Then the sample has been deformed to a set strain (50% strain). Finally, the deformed sample has been cooled down below the room temperature while keeping the strain locked-in. This stage deforms the sample into a ‘reconfigured’ state. In the second stage the deformed sample is actuated while the machine is set to prevent strain recovery in the sample. On DMA, this test was performed in the iso-strain mode (the material is given a certain amount of constant strain and the force required to maintain that strain is measured as a function of temperature). During the strain recovery test, the temperature was ramped at 5°C/min between 30° C and 100°C.
Stress Relaxation Tests: The stress relaxation properties of SMP have been determined using RSA- III (Fig. 4). In this experiment, the force required to maintain a constant tensile strain of 0.4% was monitored as a function of time, at a constant temperature of 30 °C. The gap between the clamps was kept 15 mm, as before.
Fourier Transform Infrared (FTIR) Spectra: Fourier transform infrared spectra were obtained using an Attenuated -Total- Reflectance (ATR) cell covering the 650 to 4000 cm-1 spectral range using Bruker-Tensor- 27 FTIR system. Each FTIR-ATR spectrum was the average of 64 scans at 4cm-1 of nominal spectra resolution, using air as reference. The FTIR data were analyzed using OPUS- 6.5 data Collection Program software.
RESULTS AND DISCUSSION
This section focuses on the results obtained from various tests on the degradation of Veriflex- SMP due to water and diesel fuel. The result of the ambient scan of dry SMP is shown in Figures 5 to 7. "Dry SMP" is defined as the SMP that was equilibrated at the room temperature conditions.
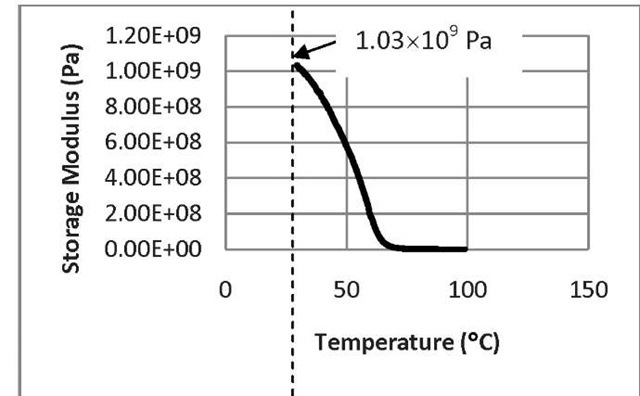
Fig. 5: The storage modulus as a function of temperature for untreated SMP
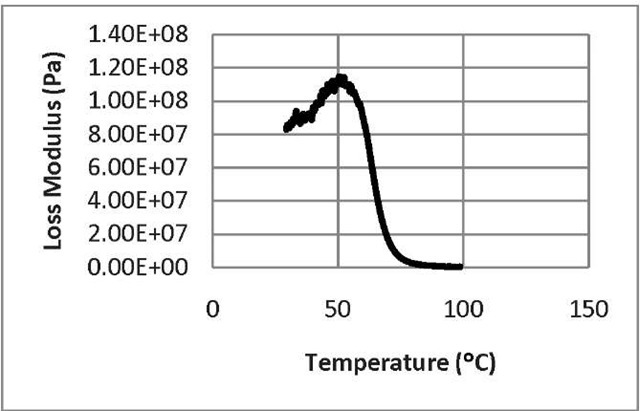
Fig. 6: The loss modulus as a function of temperature for untreated SMP
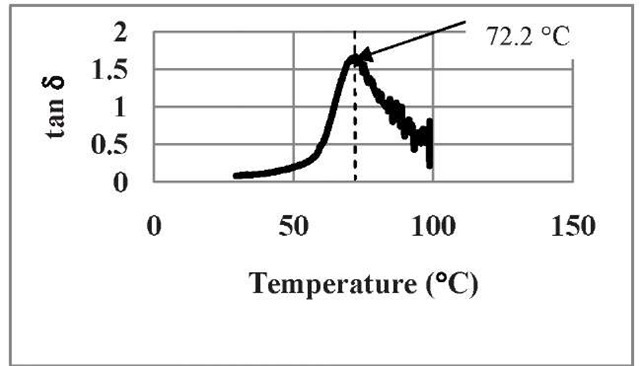
From the behavior of storage modulus curve (Fig. 5), we see that at the initial stage, the storage modulus of the polymer is 1.03 x 109 Pa which represents its load bearing capacity at room temperature whereas at high temperature, the storage modulus drops almost to zero. Macroscopically, this means that the phase angle between stress and strain approaches 90°. That is, the energy stored by the sample per cycle of deformation becomes negligible in comparison to that energy dissipated as heat. In the transition region, the storage modulus decreases with the increase of temperature. As a result, it can withstand a large amount of load. With the increase of temperature, the hard segments of shape memory polymer do not undergo any kind of change. However, the mobility of soft segments increases due to the lifting of external constraint which results in the increase of heat dissipation. As a result, the storage modulus of shape memory polymer decreases with the increase of temperature. From Fig. 6, we can see that at the beginning with the increase of temperature, the loss modulus increases. This is because with the increase of temperature, the molecular mobility of soft segment increases which, in turn, increases the heat. After going through the maximum attainable temperature limit, the loss modulus begins to decrease and ultimately, at higher temperature it goes to zero. From the behavior of tan delta curve (Fig.7), we have seen that in the transition zone between glasslike and rubberlike consistency, the tangent delta value goes through a pronounced maximum. This is associated with configurational rearrangement of the strands of the network structure of the soft segment. The storage modulus, loss modulus, and tangent delta values are the three different methods to determine the transition temperature, since each property is dependent on the molecular movement of the polymer.
Fig. 7: The behavior of tanS as a function of temperature for untreated SMP
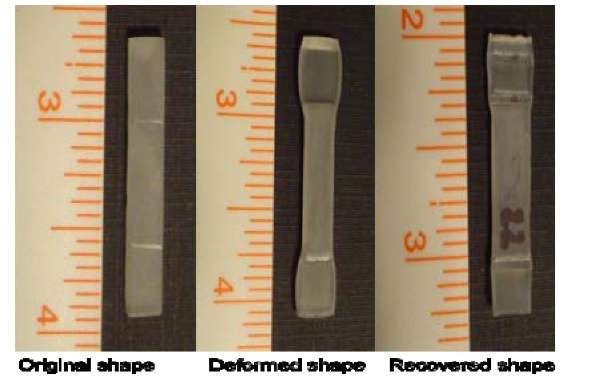
Fig. 8: Thermally driven shape memory cycle of SMP
From our results, we see that DMA spectrum of storage modulus curve of SMP shows a drop of storage modulus from 1.03 x 109 to 0.0 Pa in the temperature region between +30°C and +70° C. The loss modulus and Tan 8 shows peak at +50°C and +72.2°C, respectively. For DMA, the Tg is taken as the average of the E" and Tan 8 peak temperatures measured at 1.0 Hz. In our case, the glass transition temperature, Tg of our Veriflex-SMP, according to our test is: 61.382°C, which is close to the value 62°C supplied by the Veriflex’s manufacturer. This confirms that the correctness of our experimental procedure.
Shape Memory Property Testing:
To determine the shape memory phenomena of the SMP, the thermo-mechanical programming cycle has been done on the specimen and the results are shown in Fig. 8. The important processes are: (i) The specimen is heated to 100 °C, which is above the glass transition temperature, Tg = 62°C.; (ii) At 100 °C the specimen has been given a 50% tensile deformation by applying a tensile force on it; (iii) After the deformation, the temperature of the sample has been cooled below the room temperature while maintaining the load on the specimen; (iv) At temperature below the glass transition temperature, the load is removed and the specimen is removed from the fixture. The resulting shape is called the deformed or temporary shape. In this condition, the length of the sample has been measured. (v) Then it is heated again to 100 °C, and no-constraint was imposed on the specimen to recover the original shape; (vi) Finally, it is cooled to room temperature and the length of the sample has been measured from which the shape memory property of the sample has been calculated. The following results are obtained from the thermo-mechanical cycle on the specimen: Shape fixity (98.07%), Shape recovery ratio (96.9%), and Recovery time, 10s.