Abstract
Controlling damage progression in oxidative environments is critical for enhancing the long-term durability of polymeric resins and composites. Traditional methods for characterization of these materials for their practical service life require several thousands of hours of isothermal aging. Therefore, there is a need for accelerated testing methods in order to reduce the prohibitive cost in this testing process. Both elevated temperatures and pressure environmental conditions can accelerate oxidative aging of the materials. This paper presents methods to characterize oxidation progress and damage growth in a polymeric matrix composite based on stress-assisted diffusion and sample miniaturization. In this approach, microscale specimens are fabricated using micro-fabrication techniques. The specimens are isothermally aged at controlled stress levels that accelerate both the oxidation and damage growth in the specimen. Coupling effects of temperature and stress on the oxidative aging are investigated based on the presented method. Due to the small scale of the specimen, the number of specimens that can be tested in parallel grows significantly. Micro-fabrication techniques also allow integration of instrumentation for measurement of the specimen response during aging, thereby reducing the additional effort, time and expense of acquiring and processing the data from the specimens during the traditional long-term aging tests.
Introduction
Accelerated aging methods are needed to evaluate materials which are to be used under long-term exposure to elevated temperature in oxidative environments. The need for accelerated test methods for High Temperature Polymer Matrix Composites (HTPMC) stems from the requirements for characterization of these materials for their expected service life which can be several thousands of hours. The cost of aging these materials for this long period is often prohibitive. Thermo-oxidative stability of the HTPMC is typically determined in practice by weight loss behavior of the composites specimens. In contrast, Pochiraju, et al. [1, 2] consider the chemo-mechanics based mechanisms for modeling oxidation and damage growth in HTPMCs. In these efforts, oxygen diffusivity, rate of oxidation reaction and damage evolution kinetics of the materials were determined for high temperature resins and the oxidation behavior of the composite was simulated from the constituent behavior. Composite when subjected to oxidative environments absorbs the oxygen at the gas-solid interface and the dissolved oxygen diffuses deeper into the solid. The exposure and absorption at the surface is first of several interacting mechanisms leading to thermo-oxidative degradation. The anisotropic diffusion and the reaction of the dissolved oxygen with the polymer substrate is the mechanism driving morphological changes. The conversion of the polymer into oxidation products is generally accompanied by oxidation-driven strain and damage evolution is the next phase of oxidative degradation. The discrete damage (crack faces) form new surfaces from which oxygen is further absorbed into the structure. Damage and oxidation layer growth are strongly coupled and lead to accelerated degradation of the material.
In order to observe the long-term behavior in shorter time scales, the diffusion/reaction and damage growth behavior must be accelerated in a controlled and coupled manner. Elevated temperature and oxygen partial pressures are commonly used to accelerate the oxidation growth by speeding up diffusion and reaction behaviors. Use of stress assisted acceleration is typically complicated by the associate damage growth processes. In this paper, we examine the feasibility for miniaturization and parallelization of the oxidative aging testing, thus compressing the time required to carry out a comprehensive aging study. As the measurement of weight loss, oxidation growth and damage evolution using the methods used in previous studies [3, 4] require optical observation of morphological changes, features that assist in characterizing the oxidation and damage growth are built into the design of the specimens. The paper focuses on specimen design and fabrication techniques.
Accelerating Aging and Oxidation Processes
A good accelerated test method neither introduces extraneous damage/degradation mechanisms nor omits any actual mechanisms at its use temperature. The three primary mechanisms traditionally used for accelerating aging mechanisms are elevated temperature, elevated pressure or increased partial pressure of the oxygen in the environment and stress assisted aging.
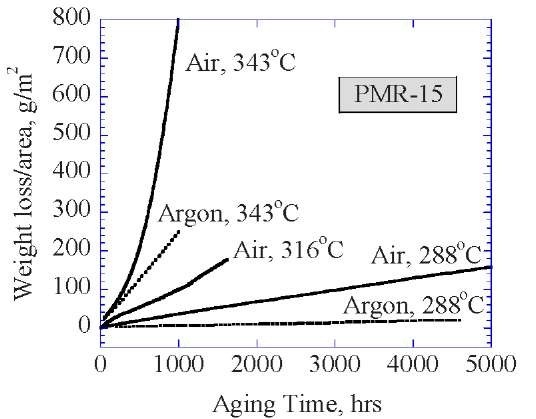
Figure 1: Weight loss in inert and oxidative environments at three temperatures
Elevated Temperature Aging
All thermally activated rate processes are accelerated by increasing the temperature. Acceleration by temperature occurs by reducing the activation energy of chemical bond rupture in the polymer macromolecule. Unfortunately, elevated temperature may promote degradation processes that do not occur at application temperatures. Temperature can also affect the rate of degradation by increasing the thermal stress in polymer composites caused by differences in the thermal expansion coefficient of the constituents. Figure 1 shows that for PMR-15 specimens aged at 288°C, which is near the use temperature, the majority of the weight loss is due to oxidation since the weight loss in an inert argon environment at this temperature is minimal. However, for specimens aged at 343°C, a substantial weight loss percentage is attributed to the non-oxidizing thermal aging (aging in an inert argon environment). The aggressive 343°C aging temperature is near the glass transition temperature of the material. Thermal aging in an inert environment is believed to involve chemical changes associated with chain scission reactions, additional cross-linking, or reduction of cross-link density, etc. that can result in changes in the molecular weight of the polymer, altering the physical and mechanical properties. Thus, there is likely a change in the thermal aging mechanism between the specimens aged at 288°C and 343°C. One therefore needs to be extremely careful in using elevated temperature for accelerated aging since the materials already operate near the Tg and one must be certain that either the aging mechanisms do not change at the elevated temperature or be able to account for changing mechanisms.
Elevated Pressure Aging
The rate of oxidation is sensitive to the partial pressure of oxygen at the composite surface and acceleration can be achieved by increasing the partial pressure of oxygen within the aging chamber. Increasing the partial pressure of oxygen can be achieved in one or two ways. Firstly the partial pressure of oxygen can be increased by increasing the mole fraction of the gas in the aging chamber. For example pure oxygen (O2) can be used instead of ambient air. However, the use of pure oxygen in a high temperature aging chamber may be a safety concern. Secondly the partial pressure of oxygen in air can be increased by increasing the total pressure since the partial pressure is directly proportional to the total pressure. By studying the dependence of the flexural strength of glass-reinforced epoxy resin on temperature and oxygen pressure, Ciutacu, et al. [5] demonstrated the importance of oxygen pressure as an accelerating factor in thermo-oxidative degradation. The results show that the same thermo-oxidative degradation mechanisms for glass-reinforced epoxy resin occur both in air and oxygen, at the pressure and temperatures used. Subsequent studies conducted by Tsotsis, et al. [6, 7] demonstrate that higher pressures of air or oxygen tend to increase the rate of degradation of polymeric composites. Recent work by Tandon et al. [8] examines the use of elevated pressure in conjunction with a realistic use temperature to accelerate the rate of thermo-oxidative degradation in PMR-15 resin. The effect of aging in 0.414 MPa (60 psi) pressured air further results in nearly a two-fold increase in the rate of volume change and increases the weight loss rate of neat resin specimens by approximately a factor of two (see Fig. 2). Further, mechanical testing reveals that the specimens aged in the pressurized air environment have by far the lowest failure strain and that the strength reduction rate is large for short aging times. Since the oxidation process in PMR-15 resin is diffusion limited versus reaction rate limited, the oxidation process is accelerated leading to significant increases in mechanical property degradation.
Stress-Assisted Aging
Figure 2: Effect of elevated pressure on oxidation behavior in a high temperature resin
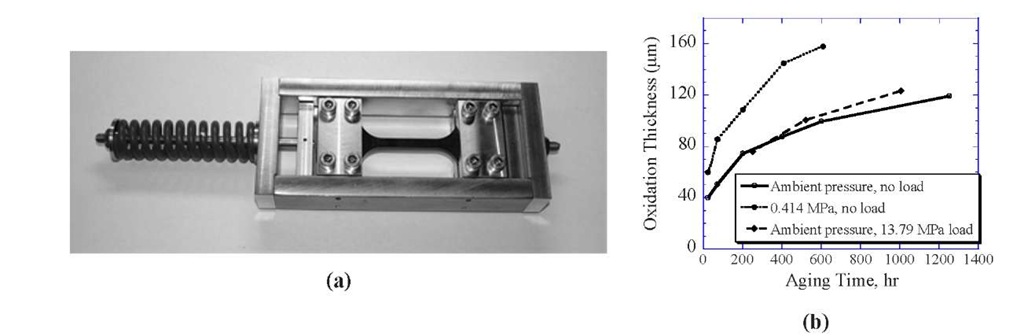
The effect of mechanical stress on long-term thermal aging was investigated in NASA’s HSR program [9]. It was observed that the addition of mechanical stress has an accelerating effect on changes in the glass transition temperature in IM7/K3B composites. However, after 10,000 hours of aging at 177°C under a constant axial load (loaded to 3000 ^in/in strain level), the axial stress applied during aging has little or no effect on aged unnotched tensile properties of quasi-isotropic lay-ups for both IM7/K3B and IM7/PETI-5 composites. To investigate the coupling effects of aging time, temperature, and stress, a low-cost pretensing fixture (inspired by the preload fixture design under the HSR program) was developed [10] that allows thermal aging of neat resin specimens under applied load at elevated temperatures. A photograph of the test fixture is shown in Fig. 3(a). The specimen is securely tightened in between the two cross-heads with the lower end kept fixed while the upper end is spring loaded to the desired stress level. The fixture assembly is then placed in the oven which is then brought up to the specified aging temperature, and the specimen is allowed to age for the specified time. Fig. 3(b) compares the total thickness of the oxidized region measured [10] in toughened polyimide neat resin at 177°C for accelerated aging environments measured using optical methods, as discussed earlier. Similar to the case of PMR-15 resin, aging under pressure [0.414 MPa (60 psi)] results in far greater oxidation zone thicknesses than are achievable in ambient air pressure environments. Additionally, using the tension aging test fixture shown in Fig 3(b) [loaded to a stress level of 13.79 MPa (2 ksi)], results in a small increase in oxidation zone thickness at longer aging times compared to an unloaded specimen in an ambient lab air environment. The stress level of 13.79 MPa is chosen based on uniaxially tension-loaded specimens tested at (177°C) to ensure that the mechanical response of the neat resin is limited to elastic behavior at the elevated temperature. Similar trends are observed for the mechanical behavior of the resin, with the tensile strength reducing considerably under accelerated pressure aging, while only minor deterioration is observed under stress-assisted aging when comparing the performance to ambient air pressure-aged specimens.
Figure 3: (a) The preloading fixture used to induce stress into the specimen during aging. (b) Effect of ambient pressure and load on oxidation growth for a polyimide.
Miniaturization and Parallelization of Stress Assisted Aging Studies
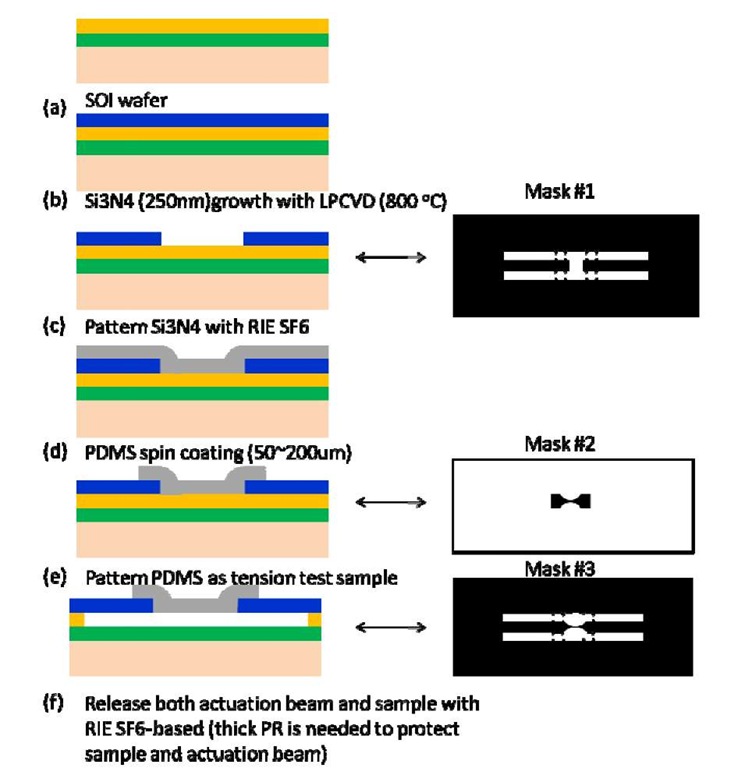
As pressure and stress-assisted acceleration of oxidation and oxidation induced damage are more effective than elevated temperature acceleration, we designed a specimen and loading frame using MEMS fabrication technology. The design concept enables introducing multiple but controlled stress levels into miniaturized specimens. The design takes advantage of the coefficient of thermal expansion mismatches to pre-strain upon the polymer tensile sample. A typical sample is fabricated using photolithography and etching processes. A sample design is shown in Figure 4. A dog-bone shaped polymer specimen (shown in gray) is attached to an actuation layer (shown in blue) and the strain is induced due to the thermal expansion mismatch at the aging temperature and process induced residual strains.
Figure 4: Geometry of the specimen and actuation beams.
The magnitude of the strain is controlled by the geometry of the actuation beam and the process parameters. The test stage consists of a pair of symmetric silicon nitride actuation beams and a test specimen in the middle. Internal stress is generated during the Low Pressure Chemical Vapor Deposition (LPCVD) of silicon nitride on the substrate. The release of the actuation beam after attaching the test specimen induces a strain upon the test specimen. This strain can be controlled by using different aspect ratios of the actuation beam and sample beam.
The pre-strain mechanism utilizes the internal stress induced from the difference in the thermal expansion coefficients of Silicon substrate and Silicon nitride during synthesis of Silicon Nitride film at high temperature (800°C) [11]. The internal stress generated is of the order of 700~800 MPa and the contraction rate of the silicon nitride actuation beam is characterized around 0.33% after release. The release step is carried out at room temperature and the high temperature polymer sample is not exposed to the LPCVD process temperatures.
The corresponding tensile loading upon the sample is given by the following equation:
"act
where![]() are, the cross section, length, mismatch strain and Young’s modulus of the actuator beam, respectively. The displacement u after the release step can be measured using the displacement of cursors embedded in the actuator and reference fixed cursors (triangular features in silicon nitride) as shown in figure 4. The value of the estimated mismatch strain is around 0.0033 [11]. The strain of test sample e can be written as:
are, the cross section, length, mismatch strain and Young’s modulus of the actuator beam, respectively. The displacement u after the release step can be measured using the displacement of cursors embedded in the actuator and reference fixed cursors (triangular features in silicon nitride) as shown in figure 4. The value of the estimated mismatch strain is around 0.0033 [11]. The strain of test sample e can be written as:
Since the interaction forces between actuator beam and with the mismatch strain for test sample of
Since the interaction forces between actuator beam and sample are equal and opposite, we have:
where a and asub are the thermal expansion coefficients of the test sample and substrate, respectively, and AT is the difference between the deposition and test temperatures. L0 and emis are the initial length and mismatch strain(developed during the deposition process) of the sample, respectively, and S is the section of the test sample.
From this equation, as the initial length and cross section of the actuator beam and test sample are known, then displacement u can be estimated. When the test sample is heated up to 177°C, due to the thermal expansion of both test sample and actuator beam,
where u and UgJt are the displacement of the test sample and the actuator beam at 177°C, respectively, and AT is the temperature change from room temperature to 177°C.
Then we can obtain the new length of the test sample and the actuator beam
Also we can calculate the final strain on the test sample
By changing the length of the test sample, different strain levels can be achieved. The relative material properties for both Silicon and Silicon nitride are listed in Table 1. In the present study, three different samples are considered with details listed in Table 2.
Table 1 Material properties of the stress-assisted oxidation stage
|
Si |
SiNx |
Polyimide |
|
|
CTE (C-1) |
2.49E-06 |
3.20E-06 |
44E-6 |
|
E (GPa) |
112.4 |
260~320 |
4.6 |
|
T (C ) |
800 |
800 |
177 |
Table 2 Sample design for three pre-strains. All dimensions are in um.
|
Test # |
Actuation beam (l x w x t) |
Sample ( l x w x t) |
Strain |
|
A |
1000 x 200×1 |
200 x 50 x 100 |
2% |
|
B |
1000 x 200 x 1 |
400x 50x 100 |
1.3% |
|
C |
1000 x 200 x 1 |
600x 50x 100 |
0.8% |
The miniaturized size of the specimens and the loading mechanism enables fabrication of multiple specimens on a single 100mm wafer. The aging process can be parallelized very economically as all the specimens are subjected to the same environmental conditions, (partial pressure and temperature). A typical 100 |am thick specimen will oxidize in 200 hours under ambient pressure and operating temperatures. Lowering the oxygen partial pressure can reduce the oxidation rates so that the long-term damage growth can be examined in the oxidation layers. With comprehensive planning and assistance from oxidation models [1], experiments can be designed with numerous specimens to measure both the oxidation and damage growth.
For each specimen with different pre-strain condition, the oxidation layer growth can be obtained by Scanning Electron Microscopy or nano-indentation techniques. Use of the prefabricated displacement cursors can allow real time image-based monitoring of the stiffness changes in the specimens.
Micro-Fabrication Processes
The fabrication of the wafer with multiple testing specimens requires several lithography, deposition and etching steps. The fabrication process steps for the strain actuation stage are shown in Figure 6. A SOI (silicon-on-insulator) wafer is used as the substrate. Then, symmetric thin silicon nitride actuation beams are deposited using LPCVD technique as shown in Figure 6 (b). High temperature (800 °C) is used to create the process-induced strain due to thermal expansion mismatch between the silicon nitride layer and the substrate. CHF3-based plasma is used with mask #1 to pattern the actuation beam as shown in Figure 6 (c). Then the test sample, BMI resin in the present study is machined and attached. Alternatively, the polymer can be masked, deposited and lifted-off as shown in Figure 6 (d) and (e). This method was demonstrated feasible by our experimental study. Finally, the test stage is released from the substrate by removing the sacrificial layer of silicon using hexafluoride-based RIE with mask 3 as shown in Figure 6(f).
In order to demonstrate the feasibility of this process, we successfully patterned the PDMS resin with lift off process as shown in Figure 7. The PDMS pattern deposited had thickness around 40 |am. The figure shows a 6×8 array of polymer test specimens on the wafer. By changing the mask design, the length of the specimen can be altered. The thickness of both the sample and the actuation beam can be changed by controlling the deposition process steps.
Figure 5: Typical wafer layout with a 3×3 array (3 samples x 3 Pre-strains) on a single 100 mm wafer.
Figure 6: Schematic of process and corresponding masks for fabrication of the stress-assisted oxidation samples.
Figure 7: Images of patterned PDMS test specimens on a SOI wafer as realized from process shown in Fig. 6.
Concluding Remarks
In this effort, we explore the feasibility of miniaturizing the aging test specimens using MEMS fabrication techniques and introducing controlled pre-strain into the specimens for stress assisted acceleration of the aging process. After ensuring the feasibility of the fabrication process and understanding the size and shape limitations, we considered the challenging task of attaching a HTPMC sample to the actuation beam. Work is currently in progress to realize high temperature polymers such as polyimides as the test specimens instead of the PDMS used for demonstration purposes. Machining the test samples and attaching the specimens to the substrate before the release step, and molding the test samples with the right geometry, are two options that may be feasible.
While the advantages of parallelization are readily obvious, the advantages of miniaturization or length scaling need to be ascertained. Reducing the specimen thickness leads to complete oxidation of the specimen in a shorter time interval and lead to acceleration of the onset and growth of damage processes. The large scale specimens currently being used require more than 500 hours of aging before the damage related mechanisms are observed and oxidation-damage coupling becomes evident.