Castings are impregnated for several reasons, the most obvious of which is to prevent leakage. Other important reasons for impregnating are to prevent corrosion, improve surface finish, remove sites that may lodge food particles and cause bacterial growth, and to prevent back seepage of occluded fluids. Leakage may be avoided by blocking the pores at any point along their length, whereas all of the other aims can be met only when the pores are blocked at the surface as well as in depth.
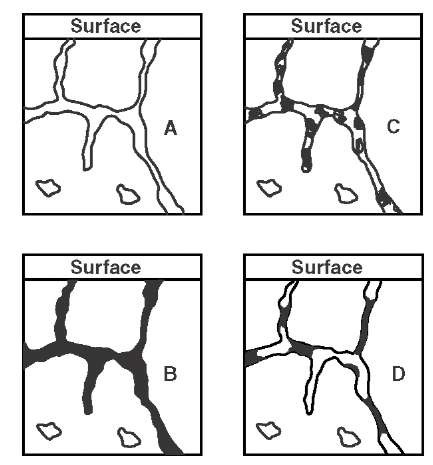
FIGURE I.1 Stages in casting impregnation process.
Impregnating Processes
Shown in Figure I.1 are the stages in the impregnation process. Figure I.1A shows a section of a porous casting with different types of porosity (continuous; non-continuous or blind; and isolated). After evacuation and impregnation under pressure, the condition shown in B (Figure I.1B) is produced. If neither bleeding nor contraction occurs before or during the curing operation, then B will also represent the final condition. However, this ideal condition is rarely attained, and some condition between B and the extreme represented by C (Figure I.1C) is more likely to occur.
Bleeding will lead to incompletely filled pores near the surface, and contraction in curing will lead to incomplete filling along the length of the pore (condition C). The latter condition may arise from loss of solvent from impregnants introduced as a solution; loss of water from vehicles such as sodium silicate; and decomposition of organic impregnants by exposure to excessive temperatures (carbonization). Some compensation for the loss of solvent or water can be obtained if the residue can be made to expand on curing, e.g., by oxidation of metallic particles carried in sodium silicate.
It has been demonstrated that the sealing action of an impregnant generally decreases as the amount of volatile in the formulation increases. One exception to this rule is demonstrated by condition D (Figure I.1D), in which a self-fluxing brazing alloy powder is fused in the pores after evaporation of the vehicle. The molten braze alloy runs into the narrowest part of the pore under capillary action and gives optimum sealing in spite of incomplete filling of the pores.
Impregnating Materials
The oldest method of reducing leakage, particularly in cast iron, is to rust or oxidize the pores, sometimes after "impregnation" with mud. Natural drying oils such as tung and linseed were also among the first impregnant materials to be used. Another early type of impregnant was based on the readily available water glass (sodium silicate).
The contraction of sodium silicate impregnants during drying was reduced by the addition of inert particles such as asbestos, chalk, or oxides. Further development has led to the so-called metallic impregnants in which a large proportion of the solids are metal powders (e.g., copper or iron). Special agents are added to the sodium silicate to reduce sedimentation. During the curing of these "metallic" impregnants, the metal particles oxidize so that a certain amount of expansion occurs to offset the considerable contraction. However, these impregnants have several disadvantages: their resistance to high temperatures is poor (e.g., 149°C max); the impregnants are attacked by steam; and the abrasive oxides make them unsuitable for bearings or machining.
There are several differences between the sodium silicate and plastics impregnants. In general, because plastics or oils do not wet castings readily, a vapor degreasing is usually necessary, followed by pumping under a vacuum of at least 712 mmHg for periods of 30 min (one impregnator has achieved success in difficult cases by holding under a vacuum of 10 |im for 2 h). A second difference results from the higher viscosity of plastics. During the pressure stage of impregnation, a minimum of 30 min under pressure may be necessary to force the plastics to flow deeply into the pores. One solution to the problem of high viscosity is to thin the plastic by solvents, but this leads to condition C.
The cost of impregnating with sodium silicate is usually less (about one quarter less) than with plastics because prior cleaning of castings needs to be less thorough and excess impreg-nant is washed away with water.
The most important types of plastics impregnants are based on the styrene monomer. A typical composition of this substitute is 80% styrene monomer with 20% linseed oil and a small quantity of organic oxidizer as a catalyst. The instability of the catalyst in the presence of lead, zinc, and copper restricts the use of this type of impregnant primarily to the light metals. The castings are heated for 2 h at 135°C to polymerize the impregnant.
The thermosetting plastics formed by copolymerization of the styrene monomer with polyesters represents an advance on the sty-rene-linseed oil polymers because it is possible to introduce liquid bath curing. The surfaces are cured rapidly when they make contact with the liquid curing agent, so that subsequent bleeding (which occurs on curing in ovens) is prevented. Other improvements that have been introduced with the styrene-polyester plastics include detergent washing (does not wash the impreg-nant from pores), freedom from inhibition by copper, and low contraction on curing (e.g., 6 to 7%). A typical curing cycle is 1 h at 135°C. These impregnants will withstand 260°C for short periods and 204°C in continuous service.
Phenolics, epoxy, and furfural plastics can also be used for impregnation. The phenolics are dissolved in a solvent such as alcohol, and require curing in two stages; the first to remove the alcohol (e.g., 1 h at 80°C) and the second to cure the plastic (e.g., 3 h at 190°C). Phenolic resins do not have good adhesion to metal and they seal according to condition C. Therefore, they do not provide the highest-quality seals, although they may be suitable for many types of work. Epoxy resins have good adhesion to metals and resistance to chemicals, but many have to be thinned with solvents to achieve low viscosity so that the impregnation occurs readily. Furfural type plastics have been developed for resistance to alkalies.
Polyester-styrene and sodium silicate are the most widely used impregnants. The plastics give the higher quality seal, but sodium silicate continues to be used because of its low cost.