Teeth in mammals are generally confined to the margins of the upper and lower jaws and are placed in four groups: incisors (I), canines (C), premolars (PM), and molars (M). The dental formula for a typical mammal is written simply as 13/3, Cl/1. PM4/4, M3/3 X 2 = 44. All teeth except the molars normally have a “milk” or deciduous set preceding the permanent ones. However, marine mammals, which are well adapted to water life, show various kinds ol teeth in number and shape. Among marine mammals, teeth of cetaceans are highly specialized compared with pinnipeds and sirenians. In cetaceans, odontocetes have a wide variety in number and shape of teeth, whereas mvsticetes are characterized by a complete absence of teeth. Most of odontocetes exhibit clear differences in tooth numbers and shapes from typical placental mammals. In some odontocetes, such as beaked whales, the pygmy sperm whale (Kogia breviceps). and the sperm whale (Physeter macro-cephalus), there is a reduction in the number of teeth in the jaws. Odontocetes have nearly homodont or peg-like teeth. Thus, in odontocetes the total tooth number for each side of the jaw is simply combined here as a single number (Table I).
I. Cetacea
Mysticetes are characterized by a complete absence of teeth, although teeth may be found in the fetal stage. For feeding on planktonic or micronectonic crustaceans such as krills and/or small pelagic fish, they use a row of baleen plates that project ventrally from the outer edges of the roof of the month. Odontocetes differ from mysticetes in feeding principally on fish and/or squid. The diet of odontocetes might reflect on the morphology and number of teeth. In odontocetes, there is no deciduous dentition. The tooth consists of enamel, prenatal dentine, postnatal dentine, cementum. and pulp cavity. Most phocoenids have spatulate teeth and delphmids conical teeth.
TABLE I
Number of Teeth in the Upper/Lower Jaw on Each Side”
|
Cetacea |
|
|
Globiccphala melas |
8-13 /15-18 |
|
G. macrorlujnchus |
7-8/7-8 |
|
Pseiidorca crassidens |
7-12 / 7-12 |
|
Lagcnorlujnchus obliquidens |
2:3-26/23-26 |
|
L. obscurus |
27-36 / 27-36 |
|
L. albirostris |
22-28 / 22-28 |
|
L. acutm |
30-40/30-40 |
|
L. australis |
27-33 / 27-33 |
|
L. cmciger |
26-35 / 27-35 |
|
Ttirsiops tnuicatus |
18-26 /18-26 |
|
Orcinus oral |
10-12/10-12 |
|
Grampus griseus |
0/2-7 |
|
Steno bredancnsis |
19-26 /19-28 |
|
Sousa chincnsis |
30-35/31-34 |
|
S. plumbea |
31-38 / 30-37 |
|
S. tcuszii |
27-30/27-31 |
|
Sotalia fluviatilis |
25-36 / 25-36 |
|
Stc.nclla attenuata |
35-48/34-37 |
|
S. longirostris |
44-64/42-62 |
|
S. eocndeoalba |
39-53/39-55 |
|
S. chjmene |
36-49/38-48 |
|
S. frontalis |
32-42/30-40 |
|
Delphinus delphis |
45-60/45-60 |
|
Lagcnodelphis hosei |
36-44/34-44 |
|
Cephalorlu/nehus commersonii |
28-34 / 26-35 |
|
C. eutropia |
28-34 / 29-33 |
|
C. hectori |
24-31 / 24-31 |
|
C. heavisidii |
25-28 / 25-28 |
|
Lissodelplus borealis |
37-54/37-54 |
|
L. peronii |
39-50 / 39-50 |
|
Peponocephala elcctra |
20-26/22-25 |
|
Feresa attenuata |
8-11/10-13 |
|
Neophocaena phocanoidcs |
13-22/13-22 |
|
Phococna spinipinnis |
10-23 /14-23 |
|
P. sinus |
16-22/17-20 |
|
P. phococna |
19-28/19-28 |
|
P. dioptrica |
17-23/17-20 |
|
Phococnoides dalli |
23-28 / 23-28 |
|
Monodon monoceros |
1/0 |
|
Delphinaptcrus leitcas |
9/8 |
|
Orcaella brevirostiis |
17-20/15-18 |
|
Inia geoffrensis |
24-35/24-35 |
|
Lipotes vexillifer |
30-34/32-36 |
|
Pontoporia blainvillei |
53^8/51-56 |
|
Platanista gangetica |
25-39/26-35 |
|
Physeter macrocephalus |
0/17-29 |
|
Kogia breviceps |
0/12-16 |
|
K sima |
0/8-11 |
|
Berardius bairdii |
0/2 |
|
B. arnuxii |
0/2 |
|
Ziphius cavirostris |
0/1 |
|
Tasmacetus shepherdi |
17-21 /17-29 |
|
Hijperoodon ampullaftis |
0/1 |
|
H. planifrons |
0/1 |
|
Indopacetus pacijicus |
0/1 |
|
Mesoplodon bidens |
0/1 |
|
M. bowdoini |
0/1 |
|
M. carlhubbsi |
0/1 |
|
M. dcnsirostris |
0/1 |
|
M. europaeus |
0/1 |
|
M. ginkgodens |
0/1 |
|
M. graxji |
17-22/1 |
|
M. hectori |
0/1 |
|
M. layardii |
0/1 |
|
M. minis |
0/1 |
|
M. stejnegeri |
0/1 |
|
Pinnipedia |
|
|
Phoca vitulina |
13/2, Cl/1, PC5/5 |
|
P largha |
13/2, Cl/1, PC5/5 |
|
Pusa hispida |
13/2, Cl/1, PC5/5 |
|
P. sibirica |
13/2, Cl/1, PC5/5 |
|
P. caspica |
13/2, Cl/1, PC6/5 |
|
Pagophilus groenlandictis |
13/2, Cl/1, PC5/5 |
|
Histriophoca fasciata |
13/2, Cl/1, PC5/5 |
|
Halichoeras grypus |
13/2, Cl/1, PC5-6/5 |
|
Erignathus barbatus |
13/2, Cl/1, PC5/5 |
|
Cystophora cristata |
12/1, Cl/1, PC5/5 |
|
Monachus monachus |
12/2, Cl/1, PC5/5 |
|
M. tropicalis |
12/2, Cl/1, PC5/5 |
|
M. schauinslandi |
12/2, Cl/1, PC5/5 |
|
Lobodon carcinophaga |
12/1, Cl/1, PC5/5 |
|
Ommatophoca rossii |
12/2, Cl/1, PC5-6/4-6 |
|
Hydrurga leptomjx |
12/2, Cl/1, PC5/5 |
|
Leptomjchotes weddellii |
12/2, Cl/1, PC5/5 |
|
Mirounga leonina |
12/1, Cl/1, PC5/5 |
|
M. angustirostris |
12/1, Cl/1, PC5/5 |
|
Odobenus rosmarus |
11/0, Cl/1, PC3/3 |
|
Phocarctos hookeri |
13/2, Cl/1, PC6/5 |
|
Ota ria jlavescem |
13/2, Cl/1. PC6/5 |
|
Zalophus californianus |
13/2, Cl/1, PC5-6/5-6 |
|
Neophoca cinerea |
13/2, Cl/1. PC5/5 |
|
Eumetopias jubatus |
13/2, Cl/1, PC5/5 |
|
Callorhimis ursinus |
13/2, Cl/1. PC6/5 |
|
Arctocephalus pusillus |
13/2, Cl/1, PC6/5 |
|
A. gazella |
13/2, Cl/1, PC6/5 |
|
A. forsteri |
13/2, Cl/1, PC6/5 |
|
A. tropicalis |
13/2, Cl/1, PC6/5 |
|
A. australis |
13/2. Cl/1, PC6/5 |
|
A. galapagoensis |
13/2, Cl/1. PC6/5 |
|
A. philippii |
13/2, Cl/1, PC6/5 |
|
A. toivnsendi |
13/2, Cl/1, PC6/5 |
|
Sirenia |
|
|
Dugong dugon |
12/3, C0/1, PC6/6 |
|
Trichechus manatus |
10/0, C0/0, PC5-7/5-7 |
|
T. senegalensis |
10/0. C0/0, PC5-7/5-7 |
|
T. inunguis |
10/0, C0/0, PC5-7/5-7 |
“From Ridgway and Harrison (1981, 1985. 1989. 1994) and Jefferson et al. (1993).
In most odontocetes, there is wide individual variation in tooth number. For example, in the sperm whale (Physeter macrocephalus) and the short-beaked common dolphin (Del-phinus delphis), the total number of teeth may exhibit a 1.5fold difference among individuals. Among members of the Delphinidae the number varies from 0/2 in Risso’s dolphin (Grampus griseus) to 44-64/42-62 in the spinner dolphin (Stenella longirostris). The spinner dolphin shows a wide geographical difference in number. The average highest tooth count in the lower tooth row is 45^6 in Thailand and northern Australian series, while it is 51-53 in the Central and South Pacific, western Pacific, and Philippines.
The unerupted small teeth of sperm whales remain in the upper jaw during life, as well as the pygmy sperm whale and the dwarf sperm whale (Kogia sima), and all of the lower teeth are erupted around sexual maturity. In the sperm whale the number of teeth is 0 for erupted and about 10 for unerupted teeth in each side of the upper jaw and 17-29 in the lower jaw. Pelagic dolphins such as Stenella spp. and Delphinus spp. are wide ranging in the subtropical and tropical regions of the world and are opportunistic feeders. The pincer-like action of the jaws of these long-snouted forms allows fish to be trapped by the interlocking tips of the teeth when the jaw closes and water is forced out. However, some beaked whales, the narwhal (Monodon monoceros), and Risso’s dolphin, which capture prey by suction feeding, have a relatively constant and small number of teeth.
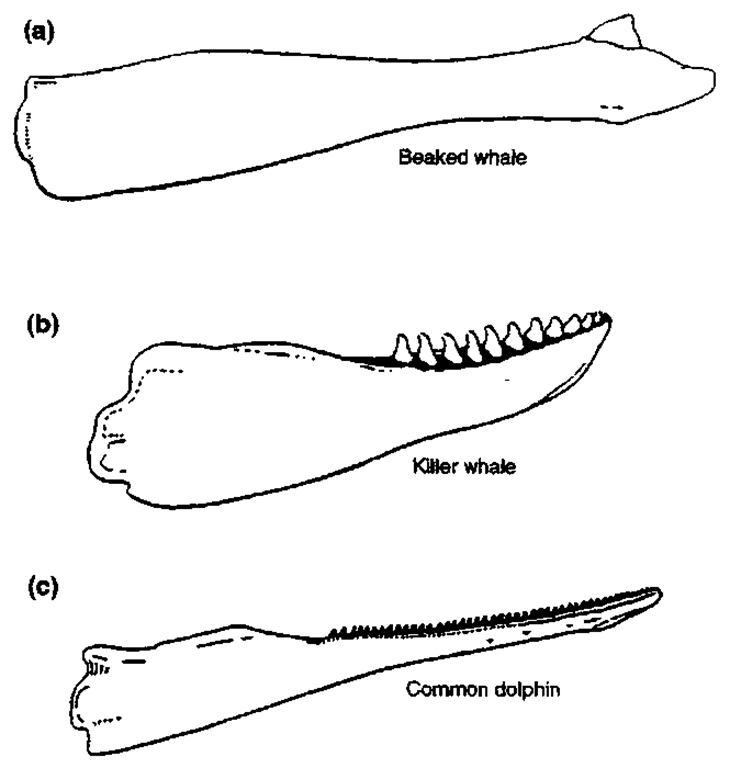
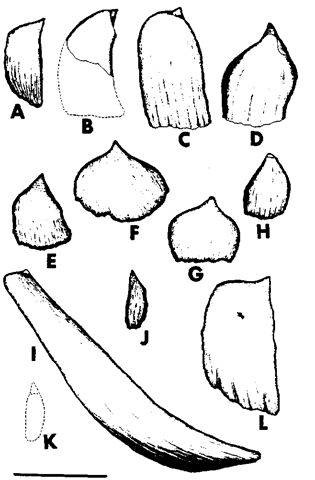
Tooth morphology also differs among species (Fig. 1). Teeth of the beaked whales are of various shapes and at various positions in the lower jaw (Fig. 2). In males, one pair of teeth in the lower jaw is erupted at attainment of sexual maturity, whereas in females they remain unerupted during life. It has been suggested that the erupted teeth may function to guide prey in the mouth, but because females and immature males lack these teeth, it is more likely that they function in intraspecific fighting. In the beaked whales of the genus Mesoplodon, maxillary teeth are absent with some exceptions. Gray’s beaked whale (Mesoplodon grayi) and Shepherd’s beaked whale (Tasnwcetus shepherdi) contain functional teeth in the upper jaw. Gray’s beaked whales have a series of 17-22 erupted small teeth in the upper jaw; however, they are not rooted in the maxilla but rather in the soft tissue over it. Some specimens of Sowerby’s beaked whale (Mesoplodon bidens) have vestigial teeth in both upper and lower jaws. Among beaked whales, the position and shape of the teeth in the mandibles of adult males are the most important key in identifying species of the genus Mesoplodon, although the shape of teeth changes with growth. In Cuvier’s beaked whale (Ziphius cavirostris), which has one pair of teeth apically, some vestigial teeth are often found in the lower jaw.
Figure 1 Comparison of representative lower dentitions of odontocetes: (a) beaked whale (Ziphiidae), (b) killer whale (Orcinus orca), and (c) common dolphin (Delphinus delphis) (Sliper, 1979, Berta and Sumich. 1999).
The Amazon river dolphin (Inia geoffrensis) has 100-140 teeth, which are conical in the anterior half of each jaw but in the posterior have a lingual flange extending from the crown. Wrinkling of the enamel makes the teeth surface rugose. In the franciscana (Pontoporia blainvillei), the crown is slender, slightly compressed anteroposteriorly. The enamel is smooth. In the Indian river dolphin (Platanista gangetica), the lower teeth are much longer than the upper, and in young animals the anterior upper and lower teeth interlock, overlapping the sides of the jaw. Although narrow and sharp in young, the teeth become worn and flattened with age.
Sperm and beaked whales exhibit a number of adaptations for employing the tongue as a piston for sucking squid into the mouth. This suction-feeding strategy is characterized by a reduced number of teeth and a small gape to allow unobstructed entry of small prey, a ribbed palate to help hold slippery-bodied squid, and throat grooves to allow for expansion of the throat region. The suction-feeding mechanism in beaked whales involves distension of the floor of the mouth provided by throat grooves and retraction of the tongue by the styloglossus and hyoglossus muscles.
Teeth are used not only for feeding but also as a symbol of social status and/or aggressive display of adult males. Tooth marks of the adult male are often found on the ventral side of Baird’s beaked whale (Berardms bairdii). In both sexes of Baird s beaked whale, the lower jaw typically bears four teeth, which are composed of a large triangular pair situated apically and a smaller peg-like pair posterior to a short diastema. The teeth erupt around the onset of sexual maturity in both sexes, with the apical pair projecting obliquely forward well beyond.
Among odontocetes, tusks of narwhal, which exceed 3 m in length and have prominent left-handed spiral ridges, are the most specialized teeth. The spiral of the tusk is sinistral (or left-handed) when viewed from the root. The tusk extends anteriorly from the head. The left anterior tooth usually erupts as a tusk only in males. However, male narwhals occasionally develop two tusks or may lack an erupted tusk, and females may develop a left tusk or both tusks. When present, the tusks of females are shorter and less robust than in males. The large disparity in size between male and female narwhals and the later attainment of sexual maturity in the male strongly suggest that the tusks are used in sexual display among males. It has been hypothesized that the tusk is used for the nonviolent assessment of social status on the basis of relative tusk size during frontal encounter, but evidence from head scarring and broken tusks indicates that adult males may indulge in aggressive displays that involve violent fighting.
Figure 2 Lateral views of right teeth of adult mule Mesoplodon: (A) M. bidens, (B) M. bowdoini. (C) M. carlhubbsi, (D) M. densirostris, (E) M. europaeus, (F) M. ginkgodens, (G) M. grayi, (H) M. hectori, (I) M. layardii, (J) M. mirus, (K) M. Pacificus, and (L) M. stenegeri (Mead, 1989). Scale bar: 10 cm.
II. Pinnipedia
Pinniped species have some reduction of teeth and milk dentition is less developed compared with typical mammals. The teeth posterior to the canines are little differentiated from one another and are referred to as postcanines (PC) or cheek teeth because molars are not clearly distinguished from premolars. Without the need for slicing or chewing while processing food (prey are swallowed whole), the cheek teeth of pinnipeds are typically homodont with a single pointed cusp for gripping slippery prey. Posterior cusps may also be present and are more frequent on posterior cheek teeth. A double-rooted condition of cheek teeth is the ancestral condition, apart from the first premolar, which is single rooted. The permanent incisors and canines normally have milk precursors, but of the postcanines, only the second, third, and fourth are preceded by milk teeth. As the permanent teeth erupt, the small alveoli gradually obliterate the alveoli of the milk teeth in the jaw.
In the walrus (Odobenus rosmarus), the formula for milk dentition is 13/3, Cl/1, PC3/3 X 2 = 28. Milk teeth are shed shortly after birth, but not all of them are succeeded by permanent teeth. Most of the functional teeth erupt in the first and second years after birth. In the adult, the formula for functional dentition is 11/0. Cl/1, PC3/3 X 2 = 18. All the teeth are simple conical crowns with a single root. The permanent upper canines empt at about 4 months of age. They have persistently open pulp cavities and continue to grow throughout the life of the animal, although the tips are abraded constantly during feeding. The upper canine teeth develop into large “tusks” in both sexes. The tusks of males are larger, thicker, spaced farther apart at the base, and more divergent at the tips than those of females, and a single tusk may reach 1 m in length (35 cm is average) and weigh as much as 5.4 kg. Male walruses use their tusks mainly in dominance displays, females use thein to defend themselves and their young, and they are occasionally used by both sexes to pull themselves onto ice floes. Tusks are not usually used in feeding, although rarely they are used to stab seal prey. The abrasion to the tips is the result of the tusks being scratched by sediment as an animal is searching for niol-lusks. its primary prey.
In Otariidae, the retention time of milk teeth depends on when the pups bite floating and other objects, but most of the milk teeth are shed by 4-5 months after birth, although there is some individual variation. Otariids have more teeth than phocids and both have more than the walrus (Table I). The permanent teeth of otariids are less diverse in morphology than those of pliocids. The typical otariid dental formula is 13/2, Cl/1, PC6/5, with some interspecific differences and individual variation.
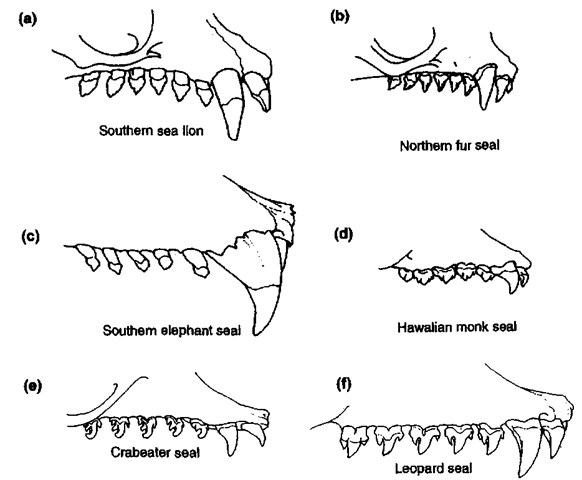
Among Phocidae. phocines have incisors 3/2 (except for die hooded seal, Cystophora cristata, which has 2/1) and monachines have incisors 2/2 (except for the elephant seals, Mirounga spp., which have 2/1). In almost all phocids species the rest of the dental formula is Cl/1, PC5/5. There is considerable variation in tooth shape and degree of cusp development (Fig. 3). Cheek teeth of the crabeater seal (Lobodon carcinophaga) and. to lesser extent, of the leopard seal (Hydmrga leptonyx) are highly modified with complex elongated cusps to trap and strain krill. Both crabeater and leopard seals are thought to suck krill into their open months by retracting their tongues and then forcing excess water out through the sieving cheek teeth. Leopard seals also possess well-developed canines for preying on penguins, other seabirds, and other pinnipeds.
III. Sirenia
The dugong (Dugong dugon) has a fixed dental formula of 12/3, CO/1. PM3/3, M3/3 with vertical replacement as in most other mammals. Dugong dentition is distinctly different from that of manatees, which lack incisors and have a different pattern of cheek tooth replacement. Two pairs of upper incisors are small and present in the juvenile dugong. Lower incisors and a canine are vestigial teeth. The deciduous (first) incisors are small, do not erupt, and are resorbed. In males, the deciduous incisors are lost around the time of tusk eruption, and their sockets disappear as the tusks expand. The tusks of males erupt at around 12 to 15 years of age and may be up to 15 cm in length with protrusion of a couple of centimeters. In females. small partially resorbed incisors may persist until the animal is about 30 years old. The permanent incisors (tusks) develop in both sexes but only erupt in males and some old females. In some old females the tusks erupt and wear, indicating that tusk eruption is not always diagnostic of the males. There are six cheek teeth (three premolars and three molars) in each jaw during the life of the dugong. These three premolars are erupted at birth and fall out because the roots of die anterior teeth are progressively resorbed. Their sockets then become occluded with bone. The molars progressively erupt during growth. The second and third molars continue to grow so that the occlusal area of the cheek teeth is maintained and increased even after the loss of anterior cheek teeth.
In the manatees (Ttichechus spp.) there are usually five to seven functional teeth in the upper and the lower jaws. Cheek teeth are replaced from the rear and fall out, as anterior teeth wear from the excessive amount of sand and grit taken during feeding on plants. The total number of teeth per jaw is estimated at 20-30 during the life of an individual. Cheek teeth are brachy-odont, or short crowned, and are enameled but lack cementum. There are two vestigial incisors in each jaw at birth, but they are later resorbed. It was thought that tooth replacement in the manatee occurs throughout the life of die individual, but there is evidence that diis may not be the case. Teedi are replaced from the rear and, as anterior teeth wear they fall out. The increase in solid food intake after weaning, and hence more chewing, acts as a mechanical stimulus for die initiation and continuation of tooth row movement. Although it is sometimes incorrectly compared to elephants, this combination of horizontal movement with an apparent limidess supply of supemumary (extra) molars is found in no other mammal, including the dugong.
Figure 3 Comparison of representative otariid (a and b) and phocid (c, d, e, and f) upper dentitions (King, 1964; Berta and Sumich, 1999): (a) Otaria flavescens, (b) Cal-lorhinus ursinus, (c) Mirounga leonina, (d) Monachus schauinslandi, (e) Lobodon car-cinophaga, and (f) Hydrurga leptonyx.
IV. Age Determination of Marine Mammals
To understand die life history of aquatic animals, age determination is a fundamental factor. Techniques for age determination of animals vary widely among species and among investigators. One of more reliable methods involves determining an individual’s age using teeth, mandibles, and ear bones. As an animal grows, incremental layers accumulate in the above hard tissues. These incremental growth layers, which are analogous to rings in tree trunks, are used for age determination of animals. Thus, counting these layers is now widely used to estimate ages of marine animals. Teeth typically are obtained from dead specimens and from captive or temporarily restrained individuals of all groups of marine mammals except mysticetes, in which growth layers in the large waxy ear plugs, as well as tympanic bullae and skull bones, have been used. Procedures for enhancing the visibility of growth increments usually include thinly slicing and polishing teeth and then etching or staining the polished surface to better resolve the growth layers. Each countable unit of repeating incremental growth layers contains at least one change in tissue density, hardness, or opacity and is referred to as a growth layer group (GLG). In most species examined so far, each GLG is thought to represent an annual increment. Useful methods for age determination by teeth are summarized as (1) preparation of unstained longitudinal thin section, (2) preparation of decalcified and stained longitudinal thin section, (3) etched preparation of the surface of the sanded half-tooth, (4) preparation of scanning electron microscopy, and (5) preparation of microradiography. The latter two methods are not convenient for mass preparation in a short time. The etched preparation of a longitudinally sectioned tooth is a good method for larger dolphins with larger teeth, and thin undecalcified sections are also useful if the longitudinal section is cut accurately or ground to include the largest possible longitudinal section of the pulp cavity. However, such material is suitable for dentinal counts only. Although the number of cemental growth layers is almost the same as the number of dentinal growth layers for young animals, the former exceeds the latter for old ones.
Cemental growth layer groups should be counted in very carefully prepared specimens of the decalcified and stained tooth. Preparation of the decalcified tooth in 5% formic acid for 24 hr and staining with Mayer’s hematoxylin solution for about 30 min of a longitudinally thin section (10-20 |xm) gives better results for small delphinid teeth.