Dimorphism means two forms. “Sexual dimorphism” * means that the two sexes of a species differ in external ! appearance or other features. Males and females may differ in size, color, shape, the development of appendages (such as horns, teeth, feathers, or fins), and also in scent or sound production. Species in which males and females are identical in appearance or other features are said to be “monomor-phic.” This article describes the types of dimorphic traits found in marine mammals and explains some of the reasons why these traits might have evolved and what can be inferred about the lives of males and females in a particular species from the pattern of sexual dimorphism. The quality of the information available on sexual dimorphism varies widely across marine mammal species. We know quite a lot about a few species, which are used repeatedly as examples, and virtually nothing about others. Despite the technical difficulties of studying marine mammals, our understanding of the evolution of sexual dimorphism is increasing steadily as observations of rarely encountered species accumulate and new techniques are developed.
I. Evolution of Sexual Dimorphism
Sexual dimorphism has fascinated biologists since before the time of Darwin. Darwin considered that most sexual dimorphism was due to sexual selection, in which evolutionary forces acted separately on the sexes (Darwin, 1871). For example, females might choose to mate with highly ornamented males (e.g., the peacock’s tail) or males might develop characters useful for fighting with other males to win in contests for access to females (e.g., large body size and antlers in deer). Today, these two processes are often referred to as female choice and contest competition, respectively. More recently, scientists have learned that males compete not only by physical fighting and display but also, in species where females mate with more than one male, by sperm competition within the female reproductive tract.
Although Darwin’s ideas about sexual selection have stood the test of time, some cases of sexual dimorphism seem to be best explained by natural selection. For example, males and females in some species of birds [e.g., Galapagos finches (genus Geospiza) and the extinct New Zealand huia (Neomorpha acu-tirostris)] have radically different bill morphologies that are best explained by sex differences in foraging habits (Anderson, 1994). In some species, females appear to be larger than males primarily because big mothers are better mothers (more eggs, better at defending their brood; Ralls, 1976). The emerging view is that the degree of sexual dimorphism in a species is the result of the difference between the sum of all the selective pressures (natural selection and sexual selection) affecting the male and the sum of those affecting the female.
II. Types of Sexual Dimorphism
The adult males and females of a species may differ in size, color, shape, the development of appendages (such as horns, teeth, feathers, or fins), scent, or vocalizations (Fig. 1). In marine mammals, one of the most striking sexually dimorphic characters is size. In some species, males are dramatically larger than females. For example, in southern elephant seals (Mirounga leonina), adult males (maximally at 3700 kg) weigh 4-10 times as much as the adult females (which weigh 350-800 kg). Males in some species also possess greatly enlarged teeth that are lacking in females and are used in fights between males. The best known example is the unicorn-like tusk of the narwhal (Monodon monoceros). The tusk, which is actually a greatly enlarged left upper tooth, usually erupts only in males and can grow to an extraordinary size, exceeding 3 m in length and 10 kg in weight. In some odontocete species (e.g., bottlenose whales, genus Hi/peroodon), males have greatly enlarged and densely ossified heads, which they use to rain other males during fights. In otariids, males have thick necks and massive chests that tend to be covered by a dense mane of hair. The noses of males are sometimes bizarrely modified. For example, the most distinctive feature of the male hooded seal (Cystophora cristata) is an inflatable hood and bright red nasal sac that may function in agonistic and courtship displays. The appendages of males (flippers, flukes, caudal peduncles, and dorsal fins) are sometimes greatly enlarged. The best known example of dorsal fin enlargement is seen in male killer whales (Orcinus orca; Fig. lb).
Figure 1 Types of sexual dimorphism in marine mammals, (a) Size. Adult male South American sea lions (O. flavescens) are two to three times heavier than females; males grow to 2.8 m and weights of340 kg; females to 2.2 m and 144 kg. There is extreme sexual dimorphism in body shape and pelage as well as size; males have massive necks, a broad head with a characteristically upturned muzzle, and a thick mane of long guard hairs. Photo by William Conway, (b) Dorsal fins. A pod of killer whales (O. orca), Alaska. In adult males, the dorsal fin is erect and may grow to 1.8 m in height whereas the dorsal fins of females are less than 0.7 m and distinctly falcate. Sexual dimorphism also occurs in body size, flipper size, and genital pigmentation pattern. Photo by Flip Nicklin (Minden Pictures), (c) Teeth and tusks. Dueling male nanvhals (M. monoceros), Canada. The unicorn-like tusk of the narwhal is actually a greatly enlarged left upper tooth. The tusk generally erupts only in males and may exceed 3 m in length and 10 kg in weight. Sexual dimorphism also occurs in body size, pigmentation pattern, and the shape of the flukes and pectoral fins. Photo by Flip Nicklin (Minden Pictures), (d) Noses. Threat vocalizations resonate in the greatly enlarged proboscis of adult male northern elephant seals (M. anguistirostris), Ano Nuevo, California. There is extreme dimorphism in body size and shape; males grow maximally to 4 m and 2300 kg and females to 3 m and 360-710 kg. The skin on the neck of the adult males is thick, rugose, and scarred by fights, and canine teeth are sexually dimorphic in size and shape. Males are darker brown than females. Photo by Sarah L. Mesnick. (e) Postanal hump. The postanal hump of adult male eastern spinner dolphins (S. longirostris orientalis) is exaggerated tremendously. The dorsal fin of adult males is also forward canted and the tips of the flukes curl up.
Although sexual dimorphism traditionally referred to differences in morphological traits, the sexes can also produce different vocalizations or odors or be differently colored or patterned. Among marine mammals, differences in color are usually limited to fairly minor differences in pattern or density of pigmentation. For example, in ribbon seals (Histriophoca fasciata), the banding pattern is similar in both sexes but paler and less distinct in females. There are numerous examples of sexually dimorphic vocalizations in marine mammals, such as the roars and bellows of male sea lions and fur seals (Otariidae), the songs of male humpback whales (Megaptera no-vaeangliae), and the loud clicks of male sperm whales (Physeter macrocephalus). In terrestrial mammals, males and females often produce different scents via urine, feces, or specialized scent glands. This has not been observed much in marine mammals but may well occur. It is known, for example, that male ringed seals (Pusa hispida) produce a strong odor in the breeding season. Male sea otters (Enhijdra latris) frequently investigate the anogenital areas of other otters, and male common bottlenose dolphins (Tursiops truncatus) investigate the urogenital region of possibly estrous females with their rostrums.
III. Taxonomic Distribution
A. Baleen Whales
Sexual size dimorphism is “reversed” among the 13 species of baleen whales with females attaining asymptotic lengths that are generally 5% longer than males. Baleen whales typically undertake long-distance migrations between their northern or southern feeding areas and their tropical breeding areas and may not feed while migrating or on the breeding grounds. Females have the added stress of pregnancy and lactation during the nonfeeding periods; a larger size may enable them to store more energy resources in the form of blubber to meet their greater reproductive demands.
Sexually dimorphic vocalizations are well known in humpback whales. Male humpbacks sing lengthy, elaborate songs, the function of which has been the subject of much speculation. Songs might function to attract females, signal status to other males, space males on the breeding grounds, synchronize estrus in females, or some combination of these. The humpback song is particularly intriguing because songs change over time, yet all members of a population sing similar songs at any one time. Male fin whales (Balaeoptcra physalus) have a patterned call, which has been termed a breeding display, but observations of courtship or competitive interactions are sparse. Sexually dimorphic vocalizations may also exist in blue whales (Balaenoptera musculus). There is dimorphism in the shape of the upper jaw of fin whales and, to a lesser extent, Bryde’s whales (Balaenoptera edeni), but the function of this dimorphism is unknown.
Observations of clear aggression between males are known only in humpback and southern right whales (Eubalaena aus-tralis). Thus, it is not surprising that there are few accounts of sexually dimorphic structures that might be used in contest competition. Male right whales, however, have larger and more numerous callosities (the raised thickened patches of skin on the head) than females, which may function as weapons in contests between males. Male right whales are also scarred more heavily than females.
B. Toothed Whales
The relative size of the sexes varies widely among the 70+ species of toothed whales. Males are larger than females in many species, with the most pronounced dimorphism in sperm whales, killer whales, bottlenose whales, narwhals, belugas (Delphinapterus leticas), and pilot whales (genus Globicephala). In sperm whales, for example, females reach about 11 inlength and 15 tons, whereas physically mature males are approximately 16 m long and weigh 45 tons. Females are slightly larger than males in Baird’s beaked whales (Berardius bairdii), the franciscana (Pontoporia blainvillei), the Indian river dolphin (Platanista gangetica), harbor porpoise (Phocoena phocoena), and dolphins in the genus Cephalorhynchus. Some species are monomorphic in size, including the Clymene dolphin (Stenella clymene), Atlantic spotted dolphins (Stenella frontalis), dwarf and pygmy sperm whales (genus Kogia), tu-cuxi (Sotalia fluviatilus), and some dolphins in the genus Lagenorhijnchus.
Differences between the sexes may occur in the size and shape of the head, teeth, thoracic girth, flukes, flippers, dorsal fin, caudal peduncle, postanal hump, and length of the beak. In general, males tend to have larger appendages than females, the exception being the few species in which females have longer rostra than males [both species of south Asian river dolphin, the franciscana, and the rough-toothed dolphin (Steno bredanensis)]. Dimorphism in the size and shape of the head may be a result of enlargement of the nose (in sperm whales) or the forehead [in bottlenose whales (genus Htjperoodon), pilot whales, and to a lesser extent in bottlenose dolphins] of adult males. The massive nasal complex of adult male sperm whales may be one-quarter to one-third the length of the animal and is probably used in the generation of sound. In bottlenose whales, the forehead is extremely steep and the surface becomes flattened in mature males. Dimorphism in the density of ossification of the bones in the head occurs in bottlenose whales (the cranium) and beaked whales of the genus Meso-plodon (the rostrum, which has one of the highest reported densities of any mammalian tissue). Differences between the sexes in the ossification of the head in these species are not surprising given observations of head butting between adult male bottlenose whales and heavy scarring on adult males of several beaked whale species of the genus Me.soplod.on. The sexually dimorphic pattern of scarring in Mesoplodon species is consistent with the idea that adult males use their rostrum, and the exposed teeth on the lower jaw, in fights with other males.
Dimorphism in the size, shape, and/or number of teeth is known in the narwhal, sperm whale, Cuvier’s beaked whale (Ziphius cavirostris), bottlenose whale, and in beaked whales of the genus Mesoplodon. In most of these species (exceptions being sperm whales and narwhals) the teeth erupt only in males and only at sexual maturity.
Differences between the sexes are known in flipper length (killer whales and melonheaded whales, Peponocephala electro), serration (Heaviside’s dolphins. Cephalorhynchus heavi-sidii), and shape of the trailing edge (belugas). In some species, including sperm whales and Dall’s porpoises (Phocoenoides dalli), males have deeper caudal peduncles than females, which may function to give more power to the flukes. Postanal humps (thought to be composed of muscle and connective tissue) are exhibited in mature males of several species, although they have been properly described and correlated with age and sex in only a few. The postanal hump of the male eastern spinner dolphin (Stenella longirostns oiientalis) is exaggerated tremendously (Fig. le). While the function of the postanal hump remains unknown, it has been suggested to be an anchor for externa] genitalia and may serve to enhance sexual performance. It may also serve as a visual signal that makes adult males easily recognizable. Dorsal fins may be larger and more erect in males than females, more hooked, or more forward-canted (Figs, lb and le). The most exaggerated examples of dorsal fin enlargement are seen in male killer whales and pilot whales. The significance of these differences in dorsal fin size and shape is unknown but they may serve a thermoregulatory function and/or as a visual signal. Differences between the sexes also occur in the flukes, which may be longer and broader in males, or differently shaped. In Dall’s porpoises and sperm whales, for example, the trailing edge of the flukes of males are convex, and in male eastern spinner dolphins, the tips of the flukes curl up. As is true for mammals in general, the distance between anal and genital openings of odontocetes tends to be greater in males than in females.
Sexual differences in pigmentation patterns are most common in the genital area but are also known to occur on the face, head, and body. Sexual dimorphism in genital pigment patterns is known in several species [killer whales, dolphins in the genus Cephalorhynchus and Lissodelphis, shortbeaked common dolphins (Delphinus delphis), Burmeister's porpoises (Phocoena spinipinnis), and Dall's porpoises]. Pigmentation differences may be related to sexual recognition, advertisement (for either males or females), or may help suckling young locate the teats. In most species of beaked whales, the body gets lighter in color with age. The lightening is especially noticeable in adult males and is primarily due to an accumulation of body scars, but may also be due to changes in pigmentation and, in several species, both. In Risso’s dolphins (Grampus griseus), ontogenetic lightening and an accumulation of body scars make older animals of both sexes appear almost pure white, and the pattern may be more prevalent in males. Adult male spotted dolphins (Stenella attenuata) bear conspicuous white rostrum tips, visible at a great distance. In Fraser’s dolphins (Lagenodelphia hosei), the intensity and thickness of the eye-to-anus stripe becomes more exaggerated (darker and thicker) in adult males. Another type of pigment dimorphism is the occurrence of visible (white or nonpigmented) linear scarring, suggested to result from a lack of repigmentation of damaged tissue from the tooth rake wounds of conspecifics. In some odontocete species, both sexes exhibit heavy scarring [e.g., Baird's beaked whale (genus Be-rardius) and Risso's dolphins]. However, in others (Mesoplodon spp., the narwhal, and the sperm whale), males are scarred more heavily than females. In these species, scarring is likely the result of wounds inflicted during male fights.
At present, acoustically dimorphic calls are known only in sperm whales. However, because odontocetes produce a wide range of sounds, dimorphic acoustic signals are likely to occur in several other species as well. Because larger animals make larger sounds, it is also reasonable to expect that other sexually dimorphic species, such as pilot whales, will produce acoustically dimorphic calls.
C. Pinnipeds
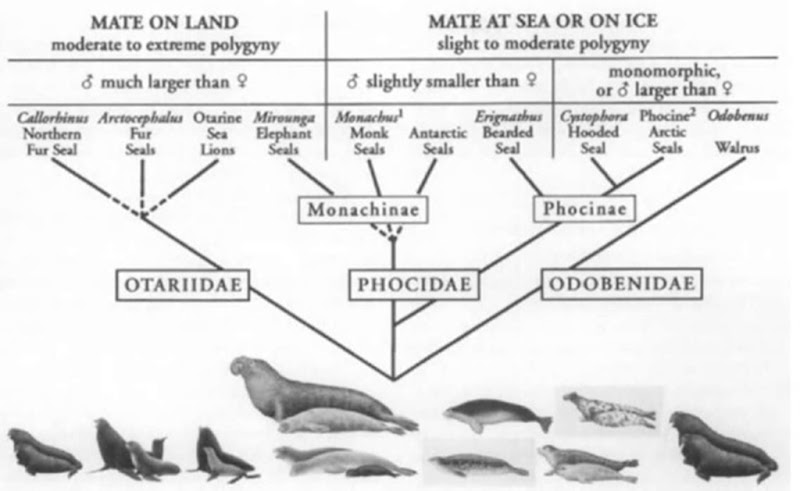
The 36 species of pinnipeds show the greatest range in sexual size dimorphism of any higher vertebrate group (Fig. 2). Adult males are up to 10 times as heavy as adult females in some species, whereas females are slightly larger than males in others. For virtually all pinnipeds studied to date, data support, or are highly suggestive of, a polygynous mating system. However, the potential for polygyny varies greatly among species and there is a strong correlation between the degree of polygyny and the degree of dimorphism. The mating system in turn, depends to a large extent on whether breeding takes place on land or at sea. In terrestrially mating pinnipeds [this includes sea hons and fur seals and three species of phocid, the northern and southern elephant seal (genus Mirounga) and the gray seal (Halichoerus grypus)], extreme polygyny is possible because females gather in dense groups on islands to give birth and mate. Under these conditions, a successful male can defend many females. In these species, males exhibit not only large size but also other characteristics useful in fights over females, such as large canines, massive necks and chests, and dense pelage. Large size may also help males of these species achieve greater reproductive success by enabling them to remain longer on the breeding rookery because larger males have bigger energy reserves in the form of blubber.
In the remaining pinnipeds, the walrus (Oclobenus ros-marus) and nearly all of the phocids, mating takes place in the water. Females of many species give birth on ice (and therefore are not as spatially clumped as terrestrially breeding species in part because they have larger expanses of suitable habitat available) and the mating season is short. Thus, males have less opportunity to defend and mate with multiple females. These characteristics inhibit the development of the extreme polygyny and sexual dimorphism found in terrestrially mating otariids and phocids. In general, males of aquatically mating species are only slightly larger than females or females may be slightly larger than males [bearded (Erignathus barbatus), Weddell (Leptonychotes weclclellii), Ross (Ommatophoca rossii), crabeater (Loboclon carcinophaga), and leopard (Hyclrurga leptonyx) seals]. The hooded seal is a notable exception, with males considerably larger than females. In ice-breeding species, large female size may help a mother provide large quantities of fat-rich milk for her pup, and because mating takes place in the water in these species, agility, rather than size or strength, may be important in male contests for females.
In addition to the sexual size dimorphism mentioned earlier, the sexes may also differ in pelage length and color, shape of the head and chest, canine development, and the pattern of scarring on the neck and chest. Adult male otariids tend to be bulkier than females and are distinguished readily by their thicker and more powerful necks and their massive chests. The head, neck, and chest of males tend to be covered by longer, rougher hairs, which gives the impression of a mane [e.g., the South American sea lion (Otaria flavescens); Fig. la]. In older males, the guard hairs are lighter in color and tinged with white, silver, or tan. Adult male California sea lions (Zalophus californianus) also develop a pronounced forehead, or sagittal crest, and adult male southern sea lions have a distinctive upturned muzzle. The skin on the necks of adult male elephant seals and gray seals is thickened and wrinkled and marked by scars from fights. In general, adult male otariids, as well as adult males in some species of phocids, tend to be more darkly pigmented than females.
Figure 2 Sexual size dimorphism in pinnipeds. A composite phylogenetic tree of the Pinnipedia on which sexual dimorphism, mating location, and degree of polygyny have been overlaid. Sexual size dimorphism varies greatly across pinniped species, and there is a strong correlation between the degree of dimorphism and the mating system. In otariids and three species of phocid (both elephant seals and the gray seal), mating takes place on land and extreme polygyny is possible because a successful male can defend many females. Males are much larger than females (2 to 10 times larger) and also exhibit other characteristics useful in fights over females, such as large canines. In the remaining pinnipeds, the walnts and nearly all the phocids, mating takes place in the water or on ice. There is less opportunity for males to mate with multiple females and agility, rather than size or strength, may be important in male contests for females. Males are equal, slightly, or moderately (up to 1.5 times) larger than females or females may be slightly (up to 1.1 times) larger than males in these species. 1Females are slightly larger than males in the Hawaiian monk seal: males are slightly larger than females in the Mediterranean monk seal 2Among phocines, the gray seal represents a notable case because it can exhibit both terrestrial and aquatic mating and males are maximally 3 times larger than females.
Many pinnipeds have sexually dimorphic vocalizations that may function to establish and maintain dominance relationships or to attract females. In some species, the sounds are amplified or resonated in the proboscis (as in hooded and elephant seals; Fig. Id) or an internal air sac (as in ribbon seals, bearded seals, and walruses). Hooded seals produce numerous sounds as they inflate and deflate their hood and bright red nasal sac in response to disturbances and as part of the courtship display. Male elephant seals also have greatly enlarged noses, and snouts of male gray seals are broader and more elongated than those of females. Males of these species establish dominance hierarchies through stereotyped visual and airborne acoustic threats and, less often, physical aggression. Male harbor, bearded, ribbon, Weddell, ringed, and harp (Pagophilus groenlandicus) seals are known for their acoustic courtship displays. Male harbor seals engage in complex hierarchical acoustical mating displays in which several subordinate males passively muzzle singing dominant males underwater. Much of the roaring and bellowing of adult male otariids is thought to intimidate rivals but acoustic displays may also be used to advertise to females. The walrus has the most elaborate courtship display of all pinnipeds involving intricate acoustic and visual components. Vocalizing adult male walruses apparently compete for females in lek-like groups in the water near ice floes on which females gather to pup and rest. Their surface vocal repertoire includes barks, whistles, and growls, and underwater vocalizations sound bell like. It is also thought that the massive tusks may play a role as a visual symbol of rank and as a display to females. Male walruses are larger than females in both body and tusk size. In marine mammals, the only well-documented sexually dimorphic scent of which we are aware occurs in the ringed seal. Male ringed seals give off a strong scent during the breeding season.
D. Sirenians, Sea Otters, and Polar Bears
Manatees (genus Trichechus) are generally monomorphic in size and appearance. Dugongs (Dugong dugon) exhibit no obvious sexual dimorphism apart from the short tusks (upper incisors), which usually erupt in adult males, although females may grow to a slightly larger size than males. Male dugongs compete for access to females by patrolling exclusive areas and engaging in threats, fights, and song. Adult male sea otters are larger than adult females. Second only to pinnipeds, polar bears (Ursus maritimns) exhibit the greatest sexual size dimorphism among mammals. Male polar bears may be over twice as heavy as females.
IV. What Can We Infer from Sexual Dimorphism?
The variation in sexual dimorphism among marine mammal taxa is striking. The sexes are visually indistinguishable in some species, whereas in others the differences between the sexes are so extreme that males and females live essentially separate lives except when they meet to mate. This rich variation in sexual dimorphism has prompted scientists to offer a variety of explanations. The various mechanisms of sexual selection—female choice, contest competition, and sperm competition— probably account for a large proportion of sexual dimorphism in marine mammals. However, some dimorphic traits may reflect ecological differences between the sexes (e.g., differences in beak length between the sexes in south Asian river dolphins). Others may be important for females and their young (e.g., larger females make better mothers or urogenital pigment patterns may highlight the mammary glands and help young to find them).
The functional significance of most sexually dimorphic traits in marine mammals remains untested, which is not surprising given the difficulty of observing, let alone experimenting on, most species. In general, the behavior of pinnipeds (which often breed where they can be observed) is better known than the behavior of cetaceans that breed at sea. However, extended observations of behavior have also been possible in a few cetacean species (e.g., bottlenose dolphins, humpbacks, and right whales) that breed close to shore. Nevertheless, we can often infer the functional significance of sexual dimorphism in species whose behavior is poorly known by analogy to well-studied species. The type and degree of sexual dimorphism and its association with other characteristics such as relative testis size and pattern of bodily scarring provide clues to the intensity of sexual selection (the skew in male mating success) in a species and the probable underlying mechanisms of sexual selection.
Based on studies of terrestrial mammals, a positive correlation is generally assumed between the amount of sexual dimorphism in a species and the deviation of the breeding system from monogamy. Thus, in polygynous species, male competition for access to females is severe and males are expected to exhibit traits, such as large size and big canines, favored in fights with other males over access to females. The correlation between sexual size dimorphism and the degree of polygyny has been shown across pinniped taxa (Alexander et al, 1979). For example, among otariids, the northern fur seal (Callorhinus ursinus) and Steller sea lion (Eumetopias jubatus) show the greatest relative dimorphism in body weight and defend the greatest number of females in their territories as compared to less dimorphic species. Within a species, a large body size has also been shown to be correlated with greater mating success (via dominance rank, endurance, and tenure on rookeries; e.g., elephant seals, gray seals). It is important to note, however, that species that lack sexual size dimorphism do not necessarily lack male-male competition for mates. In these species, sexual selection may be intense, but due to different forms of competition among males for access to mates, and the consequences may not be reflected in size but in other characters, such as song, visual display, or agility.
Sexual dimorphism in size and weaponry (big teeth, enlarged heads, and strong flukes) suggests that contest competition for access to mates plays an important role in the mating strategies of males in many marine mammal species. Contest competition may take the form of ritualized displays (visual or acoustic), by which potential rivals assess relative size or strength, or physical aggression. Among odontocetes, dimorphism in weaponry is correlated to patterns of body scarring and observations of head butting among males. In certain species, such as sperm whales, beaked whales, and narwhals, teeth erupt or are enlarged only in adult males, a pattern that suggests their function has shifted from feeding to use in social interactions. Adult males of many of these same species are heavily scarred, another trait that suggests males use their teeth in physical battles with other males.
Among terrestrial mammals, the relationship between relative testis size and mating system is so strong that relative testes size can be used as a good indicator of the mating system (Goniendio et al, 1998). In general, where copulatory frequency is high, the testes are large, and where copulation is infrequent, the testes are small. In right whales, observations of multiple males mating with single females, together with huge (1 ton) testes, strongly suggest that sperm competition is a principal mating strategy in this species, and probably also in bowhead (Balaena mtjsticetns) and gray (Eschrichtius robns-tus) whales (Brownell and Ralls, 1986). Odontocete species such as sperm whales and beaked whales that exhibit dimorphic traits associated with intense physical combat (e.g., large size, enlarged teeth) tend to have small testes. The testes of sperm and beaked whales represent less than 0.5% of the body weight, weigh only a few kilograms, and can be held in one hand. At the other extreme, species having the largest testes, suggesting the likelihood of significant sperm competition, do not exhibit the extreme dimorphic traits associated with physical combat. These species tend to be sexually monomorphic or have dimorphic traits that may be associated with agility or visual display. For example, harbor porpoises, finless porpoises (Neophocaena phocoenoides), and dusky dolphins (Lageno-rhijnchus obscurus) have testes that represent greater than 5% of their body weight. Humans, for comparison, at about the same body mass as these dephinid species, have testes of only 0.08% of body mass (Kenagy and Trombulak, 1986).
In three-dimensional habitats, agility, rather than size or strength, may sometimes determine the outcome of male contests. Agility may be useful in scramble competition for access to mates and it may function as a visual display for female choice. This may be the case in some species, such as the aquatically mating phocids, in which males compete underwater and are smaller tiian females. Among odontocetes, larger body size typically means that the male’s propulsion structures are also proportionallv larger than those of the female. The importance of speed and maneuverability is suggested by sexual dimorphism in the flippers, flukes, caudal peduncle, and dorsal fin. Tolley et al. (1995) suggested that the larger body size, caudal peduncle, flukes, and dorsal fin of male bottlenose dolphins, and the pattern of dorsal fin scarring, are consistent with males competing for access to dispersed females. Features such as flukes and dorsal fins are used for propulsion, maneuvering, and thermoregulation and in offensive or defensive encounters with other males. More power to the flukes could increase the strength of blows and greater speed could aid in the herding of females.
Traditionally, behavioral ecologists have tended to emphasize the importance of male-male competition in the evolution of exaggerated male traits. More recently, based primarily on bird data, they have found diat female choice often plays a critical role. Recordings of male song and the existence of exaggerated morphological traits that make adult males easily recognizable suggest the importance of female choice in marine mammals. The same features that appear to provide advantages in contests between males, such as large size, big canines, or deep roars, may also be used by females to select mates and/or may function to control or intimidate females (Wells et al, 1999). Whether females actually use these traits to assess males or what these traits might signal (e.g., status, fitness, or readiness) is unknown. The enlarged postanal hump of males in some dolphins and porpoises may serve an important biomechanical function for males by facilitating copulation. It may also be important as a visual signal that makes adult males easily recognizable within schools, by both females and other males. Similarly, enlarged dorsal fins, which may have a thermoregulatory function, may also serve as a visual signal. The calls of male pinnipeds may function as male displays to females, in species recognition, and in contests between males. Evidence supporting the idea of lekking in walrus and dugongs suggests an increasing role for female choice in die evolution of vocal mating displays.
Caution is warranted when making inferences about the evolution of sexually dimorphic traits. First, our knowledge of sexual dimorphism across marine mammal taxa is incomplete. There are rarely encountered species for which we have very little information about sexual dimorphism. While our understanding of morphological differences between the sexes is growing, our knowledge of acoustic and pheroinonal differences is in its infancy. As we fill in these gaps in our knowledge, our ability to understand the underlying evolutionary patterns and processes will increase. Second, a sexually dimorphic trait may have evolved for different reasons in different species. For example, among odontocetes, males are much larger than females in sperm whales, “resident” killer whales, and long-finned pilot whales (Globicephala melas), but it is unlikely that a single explanation fits all three cases. In sperm whales, adult males are solitary and roam great distances searching for females. Males possess large teeth, have massive heads, are scarred, and have been observed ramming each other head on. It is likely that large size serves male sperm whales well in contest competition over access to females. In contrast, adult male “resident” killer whales and long-finned pilot whales live in stable social groups with their maternal relatives, are not scarred, and we know of no accounts of aggressive interactions between males. In contrast to sperm whales, “resident” male killer whales and long-finned pilot whales may increase their reproductive success, not only by mating with females in other pods, but by assisting kin in their natal pods. At this point, we can only speculate about the advantages that large size confers on males of these species, but assistance in a communal foraging strategy (“resident” killer whales) and protection of the pod (long-finned pilot whales) are possibilities. Females may prefer large males as mates in all these species, but large size may confer different advantages to individuals in each of the three cases.
Despite the technical difficulties of studying marine mammals, our understanding of the evolution of sexual dimorphism is increasing steadily. New techniques, such as scoring molecular genetic markers from tissue samples, are providing insight into social structure and variance in male reproductive success. Video, acoustic recordings, and “critter cams” (small television cameras that can be mounted on individual animals) are providing exciting new data on the behavior and interactions of animals underwater. Clearly, research opportunities abound, and the prospects for increased future understanding of the abundant sexually dimorphic traits in marine mammals are bright.