Pinnipeds are unique among mammals because they feed in the marine environment and reproduce on land or ice, requiring a spatial and temporal separation of feeding from lactation. Seals stay at sea for weeks and often months at a time, yet they must spend considerable amounts of time on land. The amphibious nature of pinniped life has necessitated a wide range of physiological adaptations to life in water and on land. Pinnipeds must meet the physiological challenges of marine existence using specialized adaptations that still facilitates existence on land. This life history requires a remarkable plasticity of physiology. Broad categories of physiological adaptation include (1) aquatic locomotion, (2) apnea and diving physiology. (3) sensory physiology, (4) osmoregulation, (5) thermoregulation, (6) fasting physiology, and (7) lactation physiology.
Pinnipeds have had to overcome numerous problems associated with moving efficiently in the dense aquatic medium, and this adaptation has reduced their ability to move about on land. Otariids have hindflippers that can be turned under the body for terrestrial locomotion, whereas phocid seals cannot turn their hindflippers under the body and instead use lunging movements to get around on land.
Perhaps the most complex suite of adaptations required for making a living in the ocean is the physiology associated with breath-hold diving to foraging depths. In addition to adaptations for dealing with great pressures, pinnipeds exhibit physiological adaptation for apnea, increased oxygen storage, brady-chardia. hypoperfusion, hypometabolism, and neuronal and hormonal control of cardiac and spleen function.
The sensory systems of pinnipeds enable them to successfully navigate, forage, and communicate in a variety of environments. Seals hear and see relatively well both in the air and underwater. Because the behavior of sound and light in water is markedly different than that in air, this again requires plasticity in their sensory physiology. Ultimately, sensory physiology must provide the appropriate visual and auditory information to facilitate social interactions on land, while allowing detection and capture of prey and detection and avoidance of predators at sea. Adaptations include well-developed underwater directional hearing and visual sensitivity at low light levels.
Living in salt water poses osmoregulatory problems for pinnipeds. In addition, pinnipeds must stay in water balance during periods on shore during which they may fast completely from food or water. Because animals also lose water for evaporate cooling, osmoregulatory strategies are linked to thermoregulation.
Pinnipeds are exposed to a remarkably variable range of environmental temperatures. They are able to tolerate frigid ocean temperatures at depth as well as high amounts of thermal radiation encountered when hauled out on land. Adaptations that help pinnipeds retain heat in the ocean environment, such as thick blubber or dense fur, may also promote overheating on land. Adaptations that may play a role in thermoregulation include large body size, blubber or dense fur, countercurrent heat exchange systems, and possibly high metabolic rates.
I. Fasting Physiology
A. Lipid Utilization and Protein Sparing
Many pinnipeds fast for extended periods during their breeding season or during molting (Table I). Mating, giving birth, nursing pups, and, for some species, molting all require long periods of time on land. This is particularly true of phocid seals, which undergo voluntary periods of prolonged fasting twice a year. Adult male pinnipeds may abstain from food or water for as long as 3 months while maintaining a territory or competing for dominance rank on the breeding rookery. In many phocid species, females fast completely from food and water for over a month while delivering tremendous amounts of energy to their pups as milk. Offspring are weaned abruptly and, in many species, die pup then undergoes an extended postweaning fast before departing to sea and initiating foraging. This postweaning fast may be an important developmental time relative to the diving physiology of the offspring. In most cases, these extended fasts are associated with behaviors or processes resulting in considerable energy expenditure (e.g., combat, mating, lactation, molting). Adult animals may lose as much as 35-57% of stored body reserves during these periods (Fig. 1).
The lengths of these voluntary fasts may vary considerably. Fasts can last as long as 3 months in breeding males of both otariids and phocids. Otariid females alternate short onshore periods with foraging trips. These fasts can last from several days to 1-2 weeks postpartum. In phocid seals that fast throughout lactation, the fasting duration can be as short as 3-5 days in hooded seals (Ctjstophora cristata), whereas northern elephant seals (Mirounga angustirostris) nurse a pup for 23-30 days after an additional 1-2 weeks fasting before parturition. Unlike most other groups of animals that undergo natural fasts, activity levels remain high. Males expend energy in territorial interaction, dominance interactions, and mating behaviors. Females expend energy in agonistic encounters for breeding space, interaction with males, and for milk synthesis. Pups are also active during their fasts, making daily movements into the water and often exhibiting high movement rates.
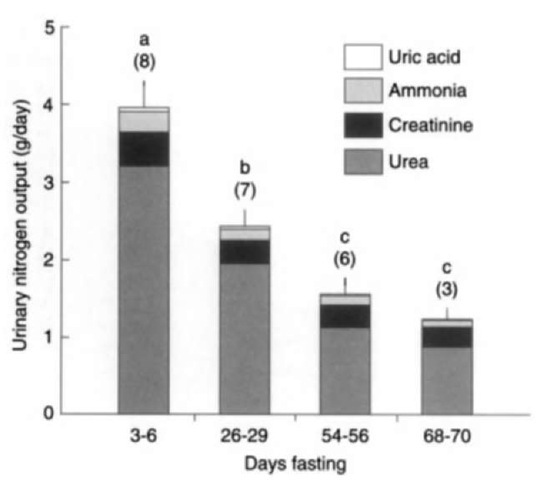
Despite this high level of energy expenditure, seals are able to minimize the depletion of lean body mass, with the bulk of energy reserves coming from adipose tissue. Within the first weeks of the fast, rates of mass loss in nonlactating animals decrease markedly and then remain relatively stable and low for the remainder of the fast. This is accomplished primarily through a reduction in metabolic rate during the fast. This decline is evident in some species on a whole body basis as well as when corrected for changing body size and composition. The key adaptation for extended fasting appears to be the ability to spare protein while fasting, thereby reducing vital organ damage. This stage of fasting, called stage II fasting, is characterized by substantial decreases in blood urea nitrogen levels and urinary excretion of nitrogenous wastes. These characteristics are evident throughout the fasts of phocid seals (Fig. 2). This decreased protein degradation is reflected in reduced absolute and proportional declines in the use of protein reserves. Protein contributes as little as 1-6% of total energy utilization by the end of the fast. For example, at the beginning of the postweaning fast, northern elephant seals meet around 4% of their energy needs through protein catabolism. By the end of the postweaning fast this value has declined to around 1%.
Nonesterified fatty acids (NEFA) provide the majority of the animal’s energy needs during long-term fasts. Increases in both turnover and plasma concentrations of NEFA have been demonstrated in several species. Reported NEFA values are greater than those reported for any other animal (as high as 3.1 mM) and increase over the fast in some species. Plasma glycerol levels show similar increases and probably are an important substrate for gluconeogenesis. There is some evidence that seals can selectively utilize reserves from different parts of the body (i.e., blubber reserves vs core tissues) during different phases of the fast. Ketone bodies (HBA) accumulate somewhat during the fast in weaned elephant seals and gray seals, Halichoerus grypus, and Weddell seals, Leptonychotes iveddellii, and subsequently decline rapidly as the end of the fast is approached. This suggests that ketone bodies may contribute to energy metabolism during long-term fasting, although levels are significantly lower than nonfasting adapted species and never reach levels affecting acid-base balance or causing keto-sis. Particularly striking in this regard are data from lactating northern elephant seals, who despite the aforementioned high and increasing NEFA levels over the fast, exhibit consistently low HBA values across lactation (0.03 to 0.13 mM). It is also important to note that increasing HBA levels were only demonstrated in juvenile animals of relatively small body size.
TABLE I
Duration of Natural Fasts for Pinnipeds Exhibiting Extended Fasts during Breeding
|
Females |
|
Males |
|
|
Crabeater seal |
~4 weeks |
Crabeater seal |
~4 weeks |
|
Gray seal |
2.5-3 weeks |
Gray seal |
3-8 weeks |
|
Hawaiian monk seal |
5-6 weeks |
Hooded seal |
~4 weeks |
|
Hooded seal |
1.5-2 weeks |
Leopard seal |
Unknown |
|
Leopard seal |
Unknown |
Northern elephant seal |
2-3 months |
|
Northern elephant seal |
5 weeks |
Ross seal |
Unknown |
|
Ross seal |
Unknown |
Southern elephant seal |
2-3 months |
|
Southern elephant seal |
4 weeks |
All fur seals |
—2.5 months |
|
Weddell seal” |
6-7 weeks |
All sea lions6 |
—2.5 months |
“Females enter the water frequently and some may feed,
California sea lion males enter the water periodically to feed. Fasting duration in the species is ~2 weeks.
Figure 1 An adult female northern elephant seal early (right) and late (left) in the lactation period. Northern elephant seal females lose between 35 and 45% of body mass daring breeding.
Figure 2 Changes in daily urinary nitrogen excretion in fasting elephant seal pups. Letters denote significant differences between periods (P < 0.05). Samples sizes are in parentheses. From Adams and Costa, J. Comp. Physiol. B 165, Water conservation and protein catabolism in northern elephant seal pups during the post weaning fast.
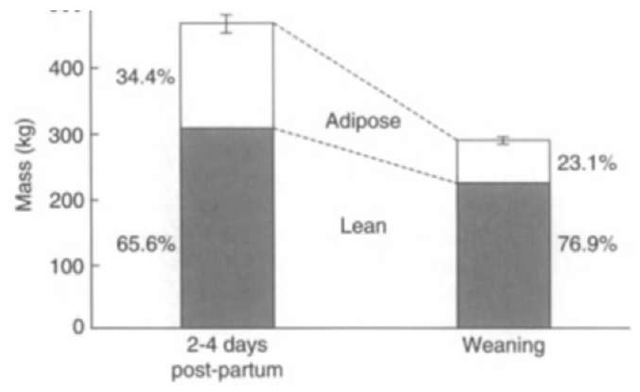
Stage III fasting or terminal starvation occurs when 30-50% of total body protein has been used. In nonfasting adapted species, this is associated with a decrease in lipid utilization and a decline in circulating ketone bodies. Evidence for entrance into stage III fasting in seals has been equivocal. The increase and subsequent decline in ketone bodies at the end of the fast in some fasting pups would suggest entry into stage III. However, only two studies have demonstrated increases in protein catabolism following the period of effective protein sparing. When considering the protein utilization by lactating females, it is important to include the loss of body protein for milk synthesis. One study on northern elephant seals demonstrated reductions in protein sparing with the depletion of lipid reserves that, together with the nutrient demands of milk synthesis, moved females close to the 30% value of body protein loss considered extreme in humans (Fig. 3). It may be that in normal, voluntary fasts, stage III fasting is never reached, with seals departing to sea before this point. Blubber reserves also play an important role in thermoregulation, and blubber depletion for energy needs is limited by the need to thermoregulate.
B. Hormonal and Fuel Regulation
Studies on hormonal and fuel regulation during fasting have suggested that seals may exhibit the protein conservation and high lipid utilization of stage II fasting throughout their lives. Fat is an important energy source throughout development and life,including high-fat milk, high-fat fish, and body fat stores. Studies of the role of insulin in glucose regulation in suckling pups have suggested that seals may be preadapted for fasting. Low insulin concentrations, impaired glucose clearance, and low insulin to glucagon ratios exhibited in both feeding and fasting pups contribute to the mobilization of lipids from body stores. In general, fasting pups are hyperglycemic and hypoinsulemic. Slow recovery of baseline blood glucose levels after insulin injection suggests that blood glucose concentrations are not closely regulated in a typical fashion by the release of the hormones insulin and glucagon. In elephant seals, insulin to glucagon ratios were less than one, which is correlated with high rates of hepatic gluco-neogenesis in other mammals. The direct contribution of glucose to die total metabolic rate is less than 1% in seals during extended fasts. Fatty acid oxidation studies confirmed diat lipid is the main energy source and suggest that glycerol liberated by lipolysis may provide substrate to meet the animal’s glucose needs. Studies on elephant seals, harbor seals (Phoca vitidina), and gray seals suggest that glucose turnover rates are typical of mammals, although glucose carbon is predominantly recycled and oxidation rates are low. Recycling protein and glucose carbon may serve as an important shuttle mechanism for carbon (e.g., synthesis of nonessential amino acids).
Figure 3 Changes in maternal mass and body composition over the lactation period in female northern elephant seals. On average, females lose 27% of total body protein stores during lactation.
Currently, our understanding of the fasting physiology of pinnipeds is biased toward studies on juvenile animals, especially the postweaning fast. Investigations have demonstrated dramatic differences in fasting physiology between lactating and molting females as well as fasting pups. The pressures of nutrient delivery for milk synthesis may have significant impacts on the metabolic strategies used by females during extended fasts. Very little is known about fasting in adult males, who potentially have some of the highest metabolic rates while fasting. Future studies can benefit from interspecific comparisons of fasting physiology relative to natural fasting durations. Even more instructive may be intraspecific comparisons among sexes and age classes during development, lactation, molting, and breeding. These comparisons will help demonstrate how the fasting biochemistry of animals responds to the varying energy and nutrient demands of breeding and lactation.
C. Renal Physiology during Fasting
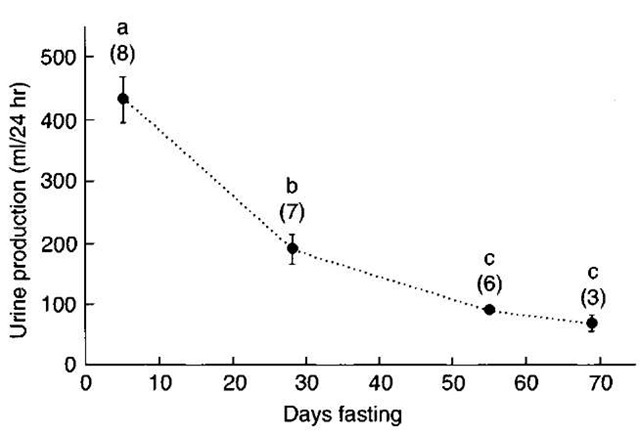
Water balance during fasting is maintained by the input of metabolic-derived water from lipid catabolism. Significant reductions in urinary water loss contribute to the maintenance of water balance (Fig. 4). The low rate of protein oxidation and an efficient urinary-concentrating mechanism in pinnipeds reduce urinary water loss during fasting. Early work on harbor seals demonstrated reductions in glomerular filtration rates (GFR) associated with fasting and hyperfiltration after feeding. Subsequent investigations have been equivocal, leaving it unclear whedier die mechanism underlying reduced urine flow is decreased glomerular filtration or increased tubular resorption. Investigations on weaned northern elephant seal pups have revealed no correlation between plasma levels of vasopressin and urinary concentrating ability. Similar investigations have demonstrated a fivefold increase in plasma aldosterone concentration during the first 5 weeks of fasting that dien began to decrease. This pattern mirrors reported changes in urine osmolality, which increases through the eighth week of the fast and dien declines. This suggests an important role for aldosterone in regulating urine concentration by its action on sodium resorption in the collecting duct.
Investigations on lactating adult northern elephant seals have demonstrated dramatic increases in GFR across the fast and suggest that these elevated rates could be an adaptation to increasing the efficiency of urea excretion during reduced urine flow. This mechanism reduces residency time and passive resorption of urea in the collecting tubules. The efficiency of urea excretion in lactating females declined from 49 to 38% over lactation, suggesting that with a declining urine flow and stable plasma urea concentration, increased GFR is necessary to increase urea excretion as protein catabolism increases.
Figure 4 The decline in daily urine production of elephant seal pups progressing through the postweaning fast. Letters denote significant differences between periods (P < 0.05). Samples sizes are in parentheses. From Adams and Costa, J. Comp. Physiol. B 165, Water conservation and protein catabolism in northern elephant seal pups during the post weaning fast.
II. Immune System
One aspect of pinniped physiology that warrants further investigation is the immune system. For the polygynous species that breed on land, breeding sites are potentially pathogenic environments. Many animals with open wounds (as high as 90% of adult males in some species) are packed closely together on a substrate soiled with excrement and rotting corpses. Neonates with poorly developed immune systems are born directly onto this substrate. Investigations have suggested depressed humoral immunity in neonates and that rapid postpartum increases in humoral immunity are the result of de novo synthesis, with a reduced role for passive maternal transfer. Interestingly, some studies have suggested increases in humoral immune response in adult females during breeding.
III. Lactation Physiology
The physiology of lactation in pinnipeds is significantly impacted by constraints resulting from the temporal separation of foraging and parental investment. Pinnipeds have evolved two general lactation strategies to manage pup provisioning within these constraints. After a short perinatal fast, otariids alternate foraging trips with suckling bouts. Initial milk production is synthesized from maternal reserves, whereas subsequent milk nutrients are derived from resources acquired while foraging. Phocids, particularly the larger species, fast during a brief but energy-intensive lactation during which nutrients for milk synthesis are derived exclusively from maternal tissues. Some smaller phocid seals, such as harbor seals or ring seals, Pusa hispida, forage during lactation. Pinnipeds consistently produce lipid-rich milk, independent of lipid content of the diet. Even more amazing is that lactation, an energetically expensive period, occurs while the female is fasting. Long-term fasting is characterized by protein sparing, reductions in metabolic rate, and reductions in water loss for urea nitrogen excretion. Studies on nonlactating fasting phocids have shown that protein stores are spared with the bulk of energy demands being supplied by the oxidation of fatty acids. In contrast, lactation is characterized by dramatic increases in metabolism and significant transfer of nutrients and water to the mammary gland for the synthesis and secretion of milk. The general metabolism of the lactating female is reorganized in a way that ensures the appropriate nutrients are partitioned to the mammary gland. In nonfasting animals, lactation is accompanied by increased levels of food consumption and digestion, with accompanying increases in the absorptive capacity of the gastrointestinal tract. In fasting phocids, regulatory mechanisms override protein and energy-sparing mechanisms to make the nutrients necessary for milk synthesis available at the expense of body nutrient reserves. The high demands of lactation coupled with complete abstinence from food and water present a complex regulatory problem. An investigation in northern elephant seals suggested that changes in the energy demands of milk synthesis across lactation may impact fasting physiology and ultimately limit the period of parental investment. From this perspective, lactating phocid females may be one of the best examples of homeorhesis “orchestrated changes for the priorities of a physiological state,” found in nature.
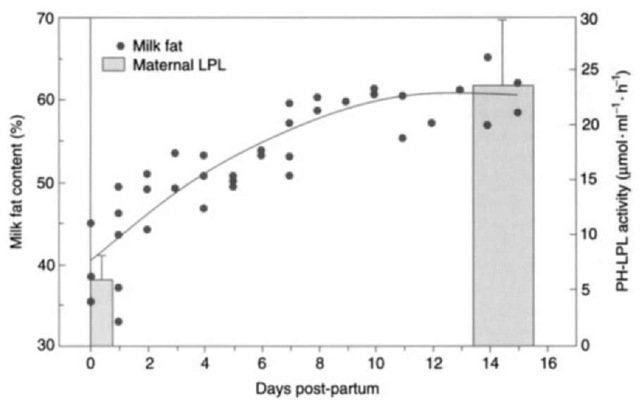
Studies have sirggested changes in milk composition and the nutrient requirements of milk synthesis across lactation in pinnipeds. These patterns are controlled by hormonal and biochemical changes. Of these changes, those that impact mobilization of adipose tissue stores, metabolism of lipids, and utilization of lipids by the mammary gland are the most significant. These changes are also important as pinnipeds transition from periods of nutrient deposition and mobilization for milk synthesis. Decreased insulin levels remove the strong antilipolytic effects of this hormone. Cortisol and other glucocorticoids influence lipid metabolism direcdy and indirectly. Cortisol stimulates hormone-sensitive lipase in adipose tissue and antagonizes the actions of insulin. Hormone-sensitive lipase activity increases lipid mobilization from adipose tissue. Lipoprotein lipase (LPL) is the primary enzyme involved in directing triglycerides mobilized from tissue stores to tissues for utilization. LPL is bound to tissues and facilitates the hydrolyzation of triglycerides, allowing uptake of fatty acids by the tissue. Under normal conditions of insulin release, LPL activity in tissues increases and triglyceride is cleared from the blood. During fasting, LPL activity in adipose tissue decreases, while hormone-sensitive lipase activity in adipocytes increases. The general pattern found in lactation is a decrease in adipocyte LPL activity before parturition and an increase in mammary gland LPL activity. The hormone prolactin is believed to be primarily responsible for LPL regulation in lactation. Investigations on harbor seals and gray seals have suggested a similar pattern. General activity levels of LPL increased 10-fold over lactation in these species and were significantly higher than levels found in humans (Fig. 5). The dramatic increase in milk lipid content early in lactation in some phocids seals may in part be explained by a significant increase in mammary gland LPL activity.
Figure 5 Changes in maternal postheparin LPL activity in relation to changes in milk fat over lactation in gray seals. From Iverson et al, J. Comp. Physiol. 165, Lipoprotein lipase activity and its relationship to high milkfat transfer during lactation in grey seals.
Very little work has been done on the physiology underlying milk secretion in pinnipeds. The release of milk fat globules occurs by apocrine secretion, in which the apical portion of the cell membrane is sloughed off. The high fat contents of pinniped milk suggest significant increases in the amount of membrane and its turnover. Pinnipeds may partially reduce this requirement by utilizing larger fat globules that require smaller amounts of membrane loss per unit lipid secreted. Data on mammary gland size in phocids have been equivocal, suggesting increased size relative to body mass in some species but not others. In any case, phocid seals appear to be particularly efficient at mobilizing and transporting nutrients to die mammary gland, perhaps by reducing the levels of de novo synthesis of milk lipids occurring at the mammary gland. This efficiency must in turn be matched by rapid and efficient digestion and assimilation of milk lipids by the of fspring.