The otters (Mustehdae; Lutrinae) provide a unique look into the evolution of marine living by mammals. This is because most extant marine mammals have been so highly modified by long periods of selection for life in the sea that they bare little resemblance to their terrestrial ancestors. Marine otters, in contrast, are recent expatriates from terrestrial and freshwater habitats, and some species still live in both environments. Contrasts within this group, and among the otters, terrestrial mammals, and the more highly adapted pinnipeds and cetaceans potentially offer deep insight into mammalian adaptations to life in the sea. Among the marine mammals, sea otters also provide the clearest understanding of predation and ocean ecosystem function. This is due in part to serendipitous opportunities provided by history and in part by the relative ease with which shallow coastal systems where sea otters live can be observed and studied. These two qualities of the otters are what make them interesting to marine mammalogy. Thus, our contribution to this volume on the marine mammals is built around these themes.
I. Evolution and Phylogeny
Mustelids radiated from primitive arctoid carnivores at the Eocene/Oligocene border. Early lutrine phylogenies were based on the morphology of fossil and extant species, from which the otters were viewed as a monophyletic group that diversified into three clades: the fish eaters (Lutra, Lontra, and Pteronura), crab eaters (Aonyx), and the sea otter (Enhydra). However, the otters probably have been under strong selection for parallel or convergent evolution, thereby confounding efforts to understand phylogenetic relationships based on morphology. Distinctive features of the three purported clades might thus have resulted from differences among their common ancestors or selective divergence resulting from different prey (fishes vs invertebrates) or habitats (the ocean vs fresh water). Patterns of brain form and function exemplify this problem in the otters.
Architectural and functional differences among otter brains correlate with their principal foraging modes: invertebrate vs fish feeding. Sensory and motor function in the brains of all mammals map mediolaterally along the prefrontal gyrus. The fish-eating otters, which require precise sensory/motor function of the mouth and facial area, have well-developed proximal regions of the prefrontal gyrus. In contrast, the invertebrate feeding otters (Enhydra and Aonyx), which require precise sensory/motor function of their forelimbs for prey capture, have more highly developed lateral regions of the prefrontal gyrus. But are these features primitive or derived? If primitive, then they might accurately reflect phylogeny; otherwise, they surely do not.
Nucleotide sequence analysis of the mitochondrial cytochrome gene was used to disassociate the confounding effects of adaptation in constructing a lutrine phylogeny for 9 of the 13 extant species (Fig. 1). These data demonstrate that earlier phylogenies based solely on morphology were grossly inaccurate. The molecular analysis indicates three primary clades, including: (1) the North American river otter (Lontra canadensis), neotropical otter (L. longicaudus), and marine otter (L.fe-lina); (2) the sea otter (Enhydra lutris), Eurasian otter (Lutra lutra), spotted-necked otter (Lutra macidicollis), cape clawless otter (Aonyx capensis), and small-clawed otter (A. cinerea)-, and (3) the giant otter (Pteronura brasiliensis). Fundamental life history differences (e.g., seasonal vs aseasonal reproduction; direct vs delayed implantation) between Eurasian and North American river otters, species once thought to have formed only after the late-Pleistocene isolation of Asia and North America, make much more sense under the molecular phylogeny. The pattern of long terminal branches and short internal branches in the phylogenetic tree further suggests rapid evolutionary radiation of the otters. Estimates of divergence time indicate that the clades containing Pteronura and Lontra had separated by the late Miocene. This phylogeny also suggests that sea otters, the only fully marine otter and the most distinctive of all otter species in terms of their morphology, physiology, and behavior, diverged recently but have taken on these different characters because of strong selection imposed by life in the sea. Lutrines appear most closely related to the extant Mustela (mink, weasels, and polecats), although whether the otters originated from one or multiple radiations of this group remains uncertain.
II. Marine Otters
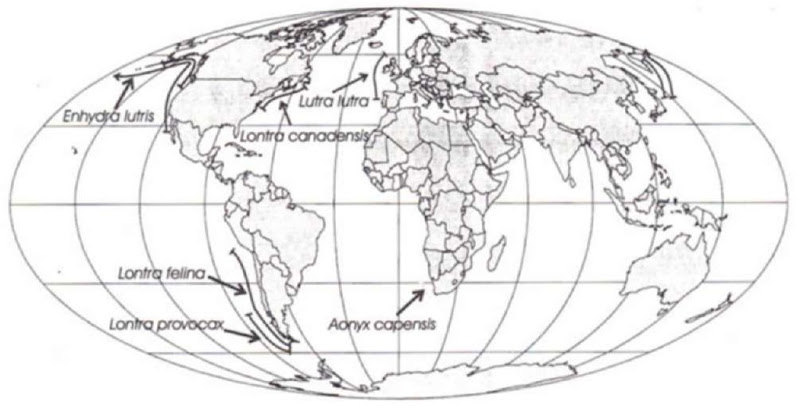
At least 6 of the 13 extant otter species are fully or partially marine living (Fig. 2). All of the marine living species or populations occur at high latitudes. Sea otters (North Pacific Ocean) and the chungungo (Lontra felina; southeast Pacific Ocean) are the only lutrines that feed exclusively in the sea. Sea otters range from the northern Japanese archipelago, across the rim of the Pacific to about central Baja California, Mexico. Chun-gungos range along the west coast of South America from Peru to southern Chile. Sea otters typically rest and give birth at sea where chungungos only enter the sea briefly to hunt. Chun-gungos often go ashore to eat their prey, rest, give birth, and rear their young in dens formed in rocky areas just above high water. Sea otters occupy a broad range of habitats, from protected bays to exposed outer shores, whereas chungungos occur only along exposed shorelines. Marine living populations of Lontra provicax occur in the protected inner waters of coastal Chile and southern Argentina. Marine living populations of North American otters occur northward from about San Francisco in the Pacific and Martha’s Vineyard in the Atlantic. Marine living populations of Eurasian otters have a roughly similar latitudinal distribution (from about Portugal northward to northern Scotland and Scandinavia in the North Atlantic; from Japan into Russia in the North Pacific). Marine living populations of cape clawless otters occur in the southernmost regions of Soudi Africa. Tropical lutrines, in contrast, rarely enter the sea.
Figure 1 Philogem of the otters. Marine otter is Lontra felina; Olingo is Bassaricyon.
While marine living species or populations of otters occur only at high latitudes, freshwater living otters occur over a much broader latitudinal range: from the most poleward ice-free environments to the tropics in the northern and southern hemispheres of both the Old and the New worlds. These distribution ill differences between marine- and freshwater-living otters probably relate to latitudinal differences in production between freshwater and coastal marine habitats, and the fact that otters have secondarily entered the sea from their primitive freshwater environments. At low latitudes, freshwater production exceeds that of the ocean, whereas at high latitudes this pattern is reversed. This production gradient may have drawn the primitively freshwater living otters into the sea at high latitudes. The most compelling evidence for the proposal is provided by Hans Kruuks comparative study of freshwater and marine living populations of Eurasian otters in northern Scotland. Kruuk found that marine living populations are able to meet their energy requirements by 2-3 hr of fishing per day, whereas those living in freshwater must double to triple the time investment in foraging to meet their energy requirements. Remarkably different population densities of marine and freshwater living otters further indicate that production and food availability are superior in coastal marine habitats. Reported densities for Eurasian and North American river otters in rivers and streams at high latitudes range between about 1 and 5 individuals per 10 km. Marine living populations of these same species are about fivefold greater, and sea otter densities range from 60 individuals per 10 km shoreline in California to >300 individuals per 10 km shoreline in the Aleutian Islands. These data indicate that otters at high latitudes maintain higher population densities in the sea than they do in freshwater and that freshwater habitats are only marginally capable of producing enough food to meet their energy requirements.
Figure 2 Geographical distributions of marine-living otters.
Different population densities of otters in freshwater and marine environments appear to have influenced the evolution of social behavior. Low-density freshwater environments afford little or no opportunity for males to compete for females; consequently, all of the freshwater living otters appear to have monogamous or promiscuous mating systems. In contrast, the elevated production and resulting high female densities may have driven sea otters to their strongly polygynous mating system.
III. Status and Trends
Except for the sea otter, there is little current information on the status and trends of marine living otter populations. The chungungo, listed in HJCN’s “Red Data,” is believed by some authorities to be threatened with extinction. Illegal harvesting for fur, habitat destruction in the form of deforestation, mining, and pollution, and competition with fisheries are thought to be the species’ main threats. Chungungos are rare from Peru southward through central Chile, but there are conflicting reports on their abundance from Cliiloe Island southward to Cape Horn.
North American river otters are common to abundant along much of the west coast of North America. Their abundance along the Atlantic coast of North America is uncertain, and information on population trends is lacking for both areas. Eurasian otters are common in marine habitats of Europe and Russia, especially from Norway to Scotland in the eastern North Atlantic. However, as for marine living river otters in North America, information on the status and trends of these populations is lacking. Coastal populations in Asia are practically unknown. The status and trends of marine living populations of southern river otters and cape clawless otters are unknown or unreported.
IV. The Sea Otter
A. History
Species resembling modem sea otters (based on body size and dentition) had arisen by the Miocene. There are two recognized lineages, one of which led to the extinct Enhydriodon and the other of which led to Enhydritherium and presumably to modem sea otters, Enhtdra spp. Members of both lineages possessed large flattened molars for crushing the exoskeletons of their invertebrate prey. Fossil remains of these early sea otters are known from North America, Eurasia, and Africa. The distribution of modem sea otters, which apparently arose during the late Pliocene or early Pleistocene, was restricted to die north Pacific Ocean (Fig. 3). One extinct species, E. macrodonta, is described from the late Pleistocene of California.
Aboriginal maritime hunters apparently depleted sea otter populations, as evidenced by the character of faunal remains in Aleut kitchen middens. However, judging from the large numbers of otters encountered by European fur hunters at the time of their first contact with aboriginal peoples, these reductions probably were limited to village sites. Sea otter populations subsequently were hunted to near extinction between 1750 and 1900 in the Pacific maritime fur trade. About a dozen remnant colonies in total containing no more than a thousand or so individuals survived into the early 20th century. Following protection in 1911, these colonies increased and several had again reached historical levels by the late 1930s or early 1940s. Rein-troductions were undertaken in the 1960s and 1970s to reestablish sea otters in Southeast Alaska. British Columbia, Washington, and Oregon, and sea otters were relocated to San Nicolas Island (southern California) in 1987. Most of the translocated populations increased at rates of 17-20% per year, about the theoretical maximum (Rmax) for sea otters. The California sea otter population has increased at a much lower rate: about 5% per year. Although precise reasons for the slow population growth in California sea otters are uncertain, the general cause is elevated mortality rather than reduced fertility. All or most of the sea otter’s range in Asia is now reoccupied. Several populations in the Kuril and Commander islands had recovered to historical levels by the 1970s or 1980s.
Figure 3 Historical and current distribution of sea otters in the North Pacific Ocean.
While sea otter populations generally have increased in number and range during the 20th century, there are exceptions. In apparent response to increased killer whale predation, sea otter populations across the western and central Aleutian archipelago declined by roughly 90% during the 1990s. The geographical extent of this decline is uncertain. Several thousand sea otters were killed by the Exxon Valdez oil spill in 1989, and while the overall impact of this event on the population is unclear, chronic detrimental effects appear to have persisted through the 1990s. The California sea otter population, which increased slowly throughout most of the 20th century, has since declined bv about 12%. The reintroduced population at San Nicolas Island, after declining sharply because of emigration, has remained roughly stable at 15-25 individuals since the early 1990s. Reintroduced sea otter populations in Southeast Alaska, British Columbia, and Washington all continue to increase in numbers and range.
B. Morphology and Physiology
Little is known about vision and hearing in sea otters. However, they are difficult to approach from upwind, thus suggesting sensitive olfaction. Sea otters appear to sense estrous females and territorial males bv moving to a downwind position from other animals. Adult male sea otters may locate estrous females by following water-borne scents. Upon entering a group of conspecifics, sea otters often perform a ritualized greeting that appears to involve scent recognition. They have a complex vocal repertoire and at least one vocal modality, the scream, is recognizably distinct among individuals.
The high thermal conductivity of water presents severe challenges to aquatic mammals. This is especially so for small species (because of high surface/volume ratios) at high latitudes or in areas of intense upwelling (because of the cold water). The high capacity for heat loss probably explains why so few marine mammal species are small. As the smallest fully aquatic mammal, sea otters face an especially severe thermal challenge because they spend all or most of their lives immersed in cold water. This challenge has somehow been met by increasing heat production or reducing heat loss. Like other mustelids, the basal metabolic rate of sea otters is well above that predicted from the Kleiber curve, and sea otters gain additional heat by the specific dynamic activity (SDA) of digestion. Sea otters lack the blubber layer that insulates cetaceans and pinnipeds, instead depending on their dense fur. Although fur in air is a superior insulator to blubber, it has three disadvantages in water-, high maintenance costs, the inability to regulate heat flow, and compressibility at depth.
In order to maintain the fur’s insulation, sea otters must groom almost continuously, an activity that consumes up to 10% of their time. Because fur (in contrast to blubber) is an inflexible insulator, sea otters require some means of facilitating heat loss during exercise. This apparently is accomplished mainly through the enlarged rear flippers, which are sparsely furred and high vascularized. The sea otter’s flippers also may absorb heat from solar radiation. The compressibility of fur causes it to lose volume, and hence insulative value, with increased depth. The rate of heat loss is doubled at a depth of about 10 m (the equivalent of two atmospheres) and is essentially unrestricted during deep dives. Water conservation also presents a challenge to sea otters because they feed primarily on marine invertebrates, which are isotonic to seawater. They meet this challenge with a large and efficient kidney.
Like all marine mammals, sea otters must dive to feed. This poses three problems: oxygen debts are incurred, with associated changes in COz concentration and pll; lactate buildups occur during prolonged, anaerobic dives; and rapid and extreme pressure forces nitrogen from the lungs into blood and other tissues, resulting in nitrogen toxicity and “the bends.” Although sea otters do not dive to such extreme depths as many other marine mammals, they are considerably better divers than other otter species, being able to attain depths in excess of 100 m. The respiratory system is appropriately modified for deep diving. For instance, the tracheal length-width ratio is less in sea otters than in river otters, thus permitting more rapid and complete air exchange before and after a dive. Like the pinnipeds, sea otters have cartilaginous airways that empty directly into their alveoli, thus ensuring patency until alveolar compression collapse during deep dives. Sea otters also have comparatively large lungs that provide both oxygen storage and increased buoyancy. Although the sea otter’s blood hemoglobin concentration is similar to that of most terrestrial mammals and less than that of most phocid seals, oxygen-hemoglobin affinity in the sea otter is relatively high, thus increasing their blood-oxygen storage capacity.
C. Locomotion
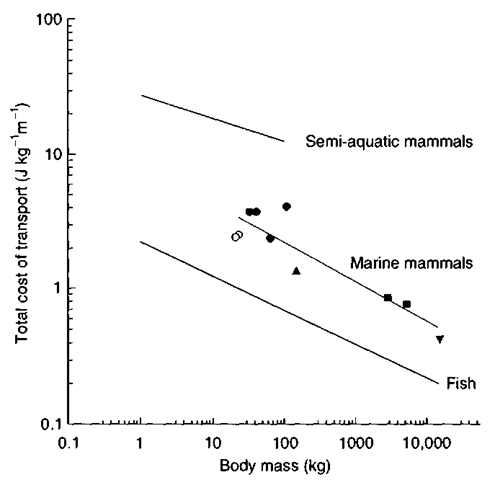
Locomotor efficiency of the semiaquatic otters is reduced on both land and in the water compared with fully terrestrial or fully aquatic mammals (Fig. 4). Among the lutrines, sea otters possess the most extreme modifications for aquatic propulsion. These include enlarged, flipper-like hindlimbs, a loosely articulated skeleton that permits increased flexibility, reduced forelimb length, and an increased tendency toward body movement and away from paddling in swimming.
The sea otter has retained a limited degree of terrestrial mobility while exhibiting morphological adaptations for improved aquatic locomotion. They rarely leave the water in some areas, whereas in others they haul out regularly. In rare instances, when sea ice prevents foraging north of the Alaska Peninsula, sea otters travel many kilometers inland in an apparent attempt to reach the Pacific Ocean.
Two types of terrestrial locomotion—walking and bounding—follow the pattern typical of terrestrial carnivores. In walking, the general motion is one of forward movement of alternate limbs in a rolling gait parallel to the long axis of the body. The head and neck are held close to the ground and the back may be horizontal, with the abdominal region contacting the ground, or the back may be arched. In some instances, sea otters pull themselves along the substrate with their fore paws. Running, described in Lontra spp. as a rapid forward movement in the same pattern as walking, has apparently been lost in the sea otter. Rapid movement of the sea otter on land is accomplished through bounding, where both forelimbs are moved forward simultaneously followed by simultaneous forward movement of the hindlimbs. During the bounding motion the back is usually highly arched. A maximum velocity on land of about 5 m/sec can be achieved in short bursts.
There are three forms of aquatic locomotion. Two of these are used predominantly while on the sea surface. One is accomplished by a sweeping motion of the tail and is used for slow movement while feeding, grooming, and maintaining its position while resting. The other, a paddling motion, consists of vertical thrusts and recovery of the hindlimbs while in either a supine or a pronate position. This type of movement is typically used during long-distance travel and may be interspersed with submerged travel, grooming, or foraging behavior. Paddling velocities range from about 0.5 to 1.0 m/sec.
Figure 4 The cost of transport for terrestrial, semi aquatic, and full aquatic mammals.
A third means of aquatic locomotion in sea otters is accomplished by craniocaudal thrusts of the pelvic limbs, often including bending of the lumbar, sacral, and caudal regions for increased speed. This movement is used in diving. While swimming, the posterior margins of the hind flippers approximate the lunate pattern and undulating movement of the caudal fin of cetaceans. The sea otter loses swimming efficiency in the resistance and turbulence during the recovery stroke and through spaces between the flippers and tail.
Travel velocities over short distances (<3 km) range from about 0.5 to 0.7 m/sec, with a maximum of about 2.5 m/sec. Estimated average rates of travel over long distances (10-75 km) range from 0.16 to 1.5 m/sec. Submerged swimming speeds average about 1.0 in/sec.
D. Diving
Diving occurs during grooming, traveling, and foraging activities. Grooming dives usually occur before feeding or resting periods and are of short duration and shallow depth. Because locomotion is more efficient underwater than on the surface, otters frequency make relatively long (30-60 sec), shallow dives while traveling between resting and feeding areas.
The characteristics of foraging dives have been described at numerous locations within the sea otter’s range. Two general types of data have been obtained: direct visual observations and remotely acquired information through radiotelemetry. Dive attributes from visual observations include dive duration, surface intervals between dives, and approximate water depths at the estimated dive locations. These data are inherently biased against animals foraging further from shore. Thus, estimates of average and maximum dive duration from animals instrumented with radio transmitters are substantially longer than those obtained visually.
Mean swimming speeds during descent and ascent in foraging dives average about 1 m/sec. In California, average dive times vary with age and sex, with maximum values estimated from juvenile males and minimum values from adult females with dependent pups. The differences likely reflect differential habitat utilization, with juvenile males foraging in deeper water offshore and females with pups foraging in shallower water nearshore. Maximum reported dive durations are 246 sec in California and 260 sec in Alaska. Dive times and their interceding surface intervals correlate with water depth, although the deepest dives are not necessarily associated with maximum dive times. Surface intervals are highly correlated with prey size and type, with the longest intervals allied with the largest prey, thus reflecting associated increases in handling and consumption times. Sea otters commonly dive to depths exceeding 40 m in the Aleutian Islands, and there is one record of a sea otter drowned in a crab pot set in 91 m of water.
Information obtained using time-depth recorders (TDRs) from Southeast Alaska further indicates individual- and gender- related differences in mean and maximum foraging dive depth distributions. Generally, adult females dive to shallower depths than adult males, although some females regularly dive to depths exceeding 60 m. Most adult males foraged at depths between 40 and 60 m, although several repeatedly dove to depths exceeding 60 m. Maximum respective dive depths were 76 and 100 m for females and males, respectively.
E. Feeding Ecology
With a few notable exceptions, sea otters forage on sessile or slow-moving invertebrates from soft sediments, rocky reefs, and kelp canopy habitats (Table I). Prey are captured mainly with the forepaws rather than in the mouth. Vision probably is used to capture highly motile prey, such as fish. However, the fact that sea otters forage at night, in highly turbid water, and at great depths suggests that vision is not a requisite sensory modality for successful feeding.
Sea otters use their enlarged molariform teeth to crush the exoskeletons of their invertebrate prey. Tool use has developed in both the sea otter and Cape clawless otter as a further aid to foraging. This is accomplished by using rocks or other hard objects to break open the exoskeletons of their invertebrate prey. Tool use is unknown in all other mammals, except primates. Tool use renders any marine invertebrate, regardless of size or shell thickness, vulnerable to sea otter predation.
Foraging for infauna requires excavation of sediments, accomplished by digging with the forepaws while offsetting the effects of neutral buoyancy with the hind flippers. Excavations for burrowing clains may be up to 1 m in depth and require displacement of large amounts of sediments. Sea otters also obtain prey by pirating it from conspecifics. Territorial males most commonly employ this method by stealing food from females that forage within their territories.
While more than 150 prey species are known to be consumed by sea otters, only a few of these predominate in the diet, depending on location, habitat type, season, and length of occupation. In California, otters foraging over rocky substrates and in kelp forests mainly consume decapod crustaceans, gastropod and bivalve molluscs, and echinoderms. In protected bays with soft sediments, otters mainly consume infaunal bivalves (Saxidomus nuttalii and Tresus nuttalltii), whereas along exposed coasts of soft sediments, Tiveb stultorum is a common prey. Important prey in Washington include crabs (species of Cancer and Pugettia), octopus (Octopus sp.), the intertidal clam (Prototheca sp.), sea cucumbers (Cucumaria miniata), and the red sea urchin (S. franciscanus). Less frequently recovered prey include the guinboot chiton (Cryptochiton stel-leri) and the purple sea urchin (S. pupuratus). The predominantly soft sediment habitats of southeast Alaska, Prince William Sound, and Kodiak Island support populations of clams that in turn are the primary prey of sea otters. Throughout most of southeast Alaska, burrowing bivalve clams (species of Saxidomus, Prototheca, Macoma, and My a) predominate in the sea otters diet, accounting for 50% of the identified prey. In Prince William Sound and Kodiak, clams account for 34-100% of the otter’s prey. Mussels (Mytilus trossulus) apparently become more important as the length of occupation by sea otters increases, ranging from 0% at newly occupied sites at Kodiak to 22% in long occupied areas. Crabs (C. tnagister) were once important sea otter prey in eastern Prince William Sound, but apparently these have been depleted by otter foraging and thus are no longer eaten in large numbers. Sea urchins are minor components of the sea otters diet in Prince William Sound and the Kodiak archipelago. In contrast, the sea otter’s diet in the Aleutian, Commander, and Kuril Islands is dominated by sea nrchins and a variety of fin fish (including hexagrammids, gad-dids, cottids, perciformes, cyclopterids, and scorpaenids). Sea urchins tend to dominate the diet of low-density sea otter populations whereas fishes are consumed in populations near equilibrium density. For unknown reasons, fish are rarely consumed by sea otters in regions east of the Aleutian Islands.
TABLE I Prey Items of the Sea Otter”
|
Prey |
California |
Oregon |
Southeast Alaska |
Aleutian Islands |
Commander Islands |
Prince William Sound |
Kodiah archipelago |
Washington |
Shuntagian Islands |
|
Echiura |
|
|
|
|
|
|
|
|
|
|
Echiunts echiunts |
|
|
X |
|
|
X |
X |
|
|
|
Urechis cattpo |
X |
|
|
|
|
|
|
|
|
|
Sipuncula |
|
|
|
|
|
|
|
X |
|
|
Nemertea |
|
|
|
|
|
|
|
|
|
|
Emplectonema sp. |
|
|
|
X |
|
|
|
|
|
|
Annelida |
|
|
|
|
|
|
|
|
|
|
Polychaeta |
|
|
|
|
|
|
|
|
|
|
Arenicola sp. |
|
|
|
X |
|
|
|
|
|
|
Eudistylia pohjmorpha |
X |
|
|
|
|
|
|
|
|
|
Eudistylia sp. |
|
|
|
|
|
X |
X |
|
|
|
Nereis sp. |
|
|
|
X |
|
X |
|
|
|
|
Nereis vexillosa |
X |
|
|
|
|
|
|
|
|
|
Arthropoda |
|
|
|
|
|
|
|
|
|
|
Crustacea |
|
|
|
|
|
|
|
|
|
|
Cirripedia |
|
|
|
|
|
|
|
|
|
|
Thoracica |
|
|
|
|
|
|
|
|
|
|
Balanus cariosus |
|
|
|
|
X |
|
|
|
|
|
B. nubiltis |
X |
|
X |
|
|
|
|
X |
|
|
Balanus sp. |
|
|
X |
|
|
|
|
|
|
|
Lepas anatifera |
|
|
|
|
X |
|
|
|
|
|
Malacostraca |
|
|
|
|
|
|
|
|
|
|
Isopoda |
|
|
|
|
|
|
|
|
|
|
Idotea sp. |
|
|
|
X |
|
|
|
|
|
|
Isopod (unidentified) |
|
|
|
X |
|
|
|
|
|
|
Amphipoda |
|
|
|
|
|
|
|
|
|
|
Amphipod (unidentified) |
|
|
|
X |
|
|
|
|
|
|
Gammants sp. |
|
|
|
|
|
|
|
|
|
|
Decapoda |
|
|
|
|
|
|
|
|
|
|
Blepharipoda occidentalis |
X |
|
|
|
|
|
|
|
|
|
Cancer antennarius |
X |
X |
|
|
|
|
|
X |
|
|
C. gracilis |
X |
|
|
|
|
|
|
|
|
|
C. nwgister |
X |
X |
X |
|
|
X |
X |
X |
X |
|
C. oregonensis |
|
|
|
X |
|
X |
|
|
|
|
C. productus |
X |
X |
|
|
|
|
X |
|
|
|
Cancer sp. |
|
|
X |
X |
|
|
X |
|
|
|
Chionccetes bairdi |
|
|
X |
X |
|
|
|
|
|
|
C. opillio |
|
|
|
|
X |
|
|
|
|
|
Cnjptolithodes sitchensis |
X |
|
|
|
|
|
|
|
|
|
Dennatunts mandtii |
|
|
|
X |
|
|
|
|
|
|
Emerita analoga |
X |
|
|
|
|
|
|
|
|
|
Hapalogaster cavicauda |
X |
|
|
|
|
|
|
|
|
|
H. grebnitzkii |
|
|
|
|
X |
|
|
|
|
|
Hemigrapsus sp. |
X |
|
|
|
|
|
|
|
|
|
Hyas coarctatus |
|
|
|
|
|
|
|
|
|
|
Lopholithodes foraminatus |
X |
|
|
|
|
|
|
|
|
|
Loxorhynchus crispatus |
X |
|
|
|
|
|
|
|
|
|
Oregonia gracilis |
|
|
|
|
|
|
|
|
|
|
Pachtjgrapsus crassipes |
X |
|
|
|
|
|
|
|
|
|
Paguristes sp. |
X |
|
|
|
|
|
|
|
|
|
Pagunts gilli |
|
|
|
X |
|
|
|
|
|
|
P. hinjstttiuscidus |
|
|
|
X |
|
|
|
|
|
|
Pagurus sp. |
|
|
|
X |
|
|
|
|
|
|
Panulints intemiptus |
X |
|
|
|
|
|
|
|
|
|
Paralithodes camtchaticus |
|
|
|
|
|
|
|
|
|
|
Placetron wosnessenski |
|
|
|
X |
|
|
|
|
|
|
Pleuroncodes planipes |
X |
|
|
|
|
|
|
|
|
|
Pugettia producta |
X |
X |
|
\ |
|
|
|
|
|
|
P. richii |
X |
|
|
|
|
|
|
|
|
Prey |
California |
Oregon |
Southeast Alaska |
Aleutian Islands |
Commander Islands |
Prince William Sound |
Kodiak archipelago |
Washington |
Shumagian Islands |
|
Pugettia sp. |
|
|
|
|
|
|
|
X |
|
|
Sclerocrangon boreas |
|
|
|
X |
|
|
|
|
|
|
Telmessus chciragonus |
|
|
X |
X |
|
X |
X |
|
|
|
Mollusca |
|
|
|
|
|
|
|
|
|
|
Gastropoda |
|
|
|
|
|
|
|
|
|
|
Astraea gibberosa |
X |
|
|
|
|
|
|
|
|
|
A. undosa |
X |
|
|
|
|
|
|
|
|
|
Argibuccinium oregonensis |
|
|
|
X |
|
|
|
|
|
|
Buccinium sp. |
\ |
|
|
X |
|
|
|
|
|
|
Calliostoma sp. |
X |
|
|
|
|
|
|
|
|
|
Crepidula adunca |
X |
|
|
|
|
|
|
|
|
|
Fusitriton oregonensis |
|
|
X |
|
|
X |
|
|
|
|
Neptunia sp. |
|
|
|
|
|
X |
|
|
|
|
Haliotis cracherodii |
X |
|
|
|
|
|
|
|
|
|
H. kamtsehatkana |
|
|
|
|
|
|
|
|
|
|
H. mfescens |
X |
|
|
|
|
|
|
|
|
|
H. walallensisa |
X |
|
|
|
|
|
|
|
|
|
Haliotis sp. |
|
|
|
|
|
|
|
|
|
|
Lottia gigantea |
X |
|
|
|
|
|
|
|
|
|
L. ochracca |
|
|
|
|
X |
|
|
|
|
|
Megathura erenulata |
X |
|
|
|
|
|
|
|
|
|
Natica clausa |
|
|
|
X |
X |
|
|
|
|
|
Notoacmaea persona |
|
|
|
|
|
X |
|
|
|
|
Polinices lewisii |
X |
|
|
|
|
|
|
|
|
|
Tectura spp. |
|
|
|
X |
|
|
|
|
|
|
Tegula biunnea |
X |
X |
|
|
|
|
|
|
|
|
T. funebralis |
X |
|
|
|
|
|
|
|
|
|
T. montercyi |
X |
|
|
|
|
|
|
|
|
|
T. pulligo |
X |
|
|
|
|
|
|
|
|
|
Tegula sp. |
|
X |
|
|
|
|
|
|
|
|
Thais sp. |
|
|
|
X |
|
|
|
|
|
|
Bivalvia |
|
|
|
|
|
|
|
|
|
|
Chlamys sp. |
|
|
|
|
|
X |
|
|
|
|
Clinocardiuin ciliatum |
|
|
|
X |
|
|
|
|
|
|
Clinocardium facantnn |
X |
|
|
|
|
|
|
|
|
|
C. nuttallii |
X |
|
X |
|
|
X |
X |
|
|
|
Entodesnw navicida |
|
|
X |
|
|
|
|
|
|
|
Gari califomica |
X |
|
X |
|
|
X |
|
|
|
|
Hiatella arctic |
|
|
|
|
|
X |
|
|
|
|
Hinnites giganteus |
X |
|
|
|
|
|
|
|
|
|
H. multirugosus |
|
X |
|
|
|
|
|
|
|
|
Hwnilaria kenerlia |
|
|
X |
|
|
X |
X |
|
|
|
Liocyim viridis |
|
|
|
X |
|
|
|
|
|
|
Macoma incongnia |
|
|
|
|
|
X |
|
|
|
|
Macoma inquinata |
|
|
|
|
|
X |
X |
|
|
|
M. nasuta |
|
|
X |
|
|
|
|
|
|
|
Macoina sp. |
|
|
|
X |
|
|
X |
|
|
|
Mactronwris polynynia |
|
|
X |
|
X |
|
|
|
|
|
Modiolus modiolus |
X |
|
X |
X |
X |
X |
X |
|
|
|
Musculus niger |
\ |
|
|
|
X |
|
|
|
|
|
M. vemicosa |
|
|
|
|
X |
|
|
|
|
|
Musculus sp. |
|
|
|
|
|
|
|
|
|
|
Mytilus californianus |
X |
X |
|
|
|
|
|
|
|
|
M. trossulus |
X |
|
X |
X |
X |
X |
X |
|
|
|
Mi/a arenaria |
|
|
X |
|
|
X |
X |
|
|
|
M. tnmcata |
|
|
X |
|
X |
X |
X |
|
|
|
Panopea generosa |
|
|
X |
|
|
|
|
|
|
|
Pecten beringianus |
|
|
|
X |
|
|
|
|
|
|
P. islandica |
|
|
|
X |
|
|
|
|
|
|
Pododesmus cepio |
X |
|
|
|
|
|
\ |
|
|
|
P. lmcroschisma |
|
|
X |
X |
X |
|
|
|
|
|
Pwtothaca staminea |
|
X |
X |
|
|
X |
X |
|
|
|
Prey |
California |
Oregon |
Southeast Alaska |
Aleutian Islands |
Commander Islands |
Prince William Sound |
Kodiak archipelago |
Washington |
Shwmgian Islands |
|
Protothaca sp. |
X |
X |
|
|
|
|
|
X |
|
|
Saxidomus giganteus |
|
|
X |
|
|
X |
X |
X |
X |
|
S. nuttalli |
X |
X |
|
|
|
|
|
|
|
|
Saxidomus sp. |
|
X |
|
|
|
|
|
|
|
|
Senipes groenlandicus |
\ |
|
X |
X |
|
X |
|
|
|
|
Siliqua patula |
X |
|
|
|
X |
|
|
|
|
|
Solen sicorius |
X |
|
|
|
|
|
|
\ |
|
|
Spisula hcmpelli |
X |
|
|
|
|
|
|
|
\ |
|
Tagelus califomiamts |
X |
|
|
|
|
|
|
|
|
|
Tivcla stultorum |
X |
|
|
|
|
|
|
|
|
|
Tresus capax |
|
|
X |
|
|
X |
X |
X |
|
|
T. nuttallii |
X |
|
|
X |
|
|
|
|
|
|
Venericardia paucicostatus |
\ |
\ |
|
X |
|
|
|
|
|
|
Voluplopsius beringi |
|
|
|
|
X |
|
|
|
|
|
Polyplacophora |
|
|
|
|
|
|
|
|
|
|
Callistochiton crassiocostatus |
X |
|
|
|
|
|
|
|
|
|
Cryptochiton stelleri |
X |
X |
X |
X |
X |
|
|
X |
|
|
Ischnochiton sp. |
X |
|
|
|
|
|
|
|
|
|
Mopalia sp. |
|
|
|
X |
|
|
|
|
|
|
Schizvplax brandtii |
|
|
|
X |
|
|
|
|
|
|
Tonicella marmorea |
|
|
|
X |
|
|
|
|
|
|
T. ruber |
|
|
|
X |
|
|
|
|
|
|
Cephalopoda |
|
|
\ |
|
|
|
|
|
|
|
Loligo opalescens |
X |
|
|
|
|
|
|
|
|
|
Octopus sp. |
X |
X |
X |
X |
|
X |
|
X |
|
|
Polypus sp. |
|
|
|
|
X |
\ |
|
|
|
|
Echinodermata |
|
|
|
|
|
|
|
|
|
|
Echinoidea |
|
|
|
|
|
|
|
|
|
|
Dendraser excentricus |
X |
|
\ |
X |
|
|
|
|
|
|
Strongltjocentrotus drobachiensis |
|
|
X |
X |
X |
X |
|
|
|
|
S. franoiscanus |
X |
X |
X |
|
|
|
|
X |
|
|
S. polyacanthus |
|
|
|
X |
X |
|
|
|
|
|
S. purpuratus |
X |
X |
X |
|
|
|
|
X |
|
|
Asteroidea |
|
|
|
|
\ |
|
|
|
|
|
Asterina miniata |
X |
|
|
X |
|
|
|
|
|
|
Ccramaster sp. |
|
|
|
X |
|
|
|
|
\ |
|
Evasterias troschelii |
|
|
|
|
|
X |
|
|
|
|
Henricia sp. |
|
|
|
X |
|
|
|
|
|
|
Leptasterias sp. |
|
|
|
X |
|
|
|
|
|
|
Pisaster brevispinus |
X |
|
|
|
|
|
|
|
|
|
P giganteus |
X |
|
|
|
|
|
|
|
|
|
P ochraceus |
X |
X |
|
|
|
|
|
X |
|
|
Pycrwpodia heliaiithoides |
X |
|
X |
|
|
|
|
X |
|
|
Solaster |
|
|
X |
|
|
|
|
||
|
Ophiuroidea |
|
|
|
|
|
|
|||
|
Brittle stars (unidentified) |
|
|
X |
X |
|
|
|
|
|
|
Gorgonocephalus cucneniis |
X |
|
X |
|
|
|
|
||
|
Holothurioidea |
|
|
|
|
|
|
|
|
|
|
Cucumtiria miniata |
|
X |
|
|
|
|
|
X |
|
|
C. piperata |
X |
|
|
|
|
|
|
|
|
|
C. fallax |
|
|
X |
|
|
|
|
|
|
|
Cucumaria sp. |
|
|
X |
X |
|
X |
|
|
|
|
Parastichopus sp. |
X |
|
|
|
|
|
|
X |
|
|
Chordata |
|
|
|
|
|
|
|
|
|
|
Ascidiacea |
|
|
|
|
|
|
|
|
|
|
Sttjefo montereyensis |
X |
|
|
|
|
|
|
|
|
|
Tunicata |
|
|
|
X |
|
|
|
|
|
|
Pisces |
|
|
|
|
|
|
|
|
|
|
Ammodytes hexapterus |
|
|
|
X |
X |
|
|
|
|
|
Anoplopoma fimbria |
|
|
|
X |
X |
|
|
|
|
|
Aptocyclus ventricosus |
|
|
|
X |
X |
|
|
|
|
|
Cottidae species |
X |
|
|
X |
|
|
|
|
|
|
Prey |
California |
Southeast Oregon Alaska |
Aleutian Islands |
Commander Islands |
Prince William Sound |
Kodiak archipelago Washington |
Shumagian Islands |
|
Cyclopteriehthxjs glaber |
|
|
X |
|
|
|
|
|
Embiotocidae species |
X |
\ |
|
|
\ |
|
|
|
Gadus tnorhua |
|
|
X |
X |
|
|
|
|
Gymnocanthus pistilleger |
|
|
X |
|
|
|
|
|
Hexagrammas superciliosus |
|
|
X |
|
|
|
|
|
Hexagraumws sp. |
X |
|
\ |
X |
|
|
|
|
Hemilepidotus hemilepidotus |
|
|
|
|
|
|
|
|
H. jordani |
|
|
|
X |
|
|
|
|
Lcpidopsetia bilineata |
|
|
|
X |
|
|
|
|
Mallotus villosus |
|
|
|
X |
|
|
|
|
Oncorhynchus nerka |
|
|
|
X |
|
|
|
|
Plewvgrammus monoterygius |
|
|
|
|
|
|
|
|
Theragra chalcograma |
|
|
|
X |
|
|
|
|
Ophiodon elongatus (egg mass) |
|
X |
|
|
|
X |
|
|
Aves |
|
|
|
|
|
|
|
|
Anatidae |
|
|
|
|
|
|
|
|
Anas crecca |
|
|
X |
|
\ |
|
|
|
Melonitta perspicillata |
X |
|
|
|
|
|
|
|
Gaviidae |
|
|
|
|
|
|
|
|
Gavia immer |
X |
|
|
|
|
|
|
|
Laridae |
|
|
|
|
|
|
|
|
Lorus sp. |
X |
|
|
|
|
|
|
|
Ph al acrocor aci dae |
|
|
|
|
|
|
|
|
Phalacrocorax sp. |
X |
|
|
|
|
|
|
|
Podicipedidae |
|
|
|
|
|
|
|
|
Aechmophoms oecidentahs |
X |
|
\ |
|
|
|
Sea otters also take advantage of episodically abundant prey. Examples include squid (Loligo sp.) and pelagic red crabs (Pleu-roncodes planipes) in California and smooth lumpsuckers (Apto-cyclus vcntricosus) in the Aleutian Islands. Pelagic red crabs appear in coastal waters of southern and central California during strong El Nino events, and vast numbers of lumpsuckers appear episodically in coastal waters of the western and central Aleutian Islands to spawn. Sea otters, on occasion, attack and consume sea birds, including teal, scoters, loons, gulls, grebes, and cormorants.
Dietary diversity increased through time as otter populations recolonized new habitats and grew toward resource limitation. Similar patterns of increased dietary diversity have been chronicled in the Aleutian Islands, Prince William Sound, and California. These changes are probably the consequence of otters reducing the abundance of their preferred prey.
Studies of marked sea otters in California have shown extreme individual variation in diet and foraging behavior. Most otters specialize on one to three prey types. This individual variation does not appear to be directly influenced by prey availability as different individuals often consume different prey at the same time and place. Dietary patterns, which appear to be inherited matrilineally, persist for years and may be lifelong characters of individuals. The causes and consequences of individual foraging patterns remain uncertain.
F. Community Ecology
1. Food Web Effects In 1960, Hairston, Smith, and Slo-bodkin proposed that “the world is green” because predators regulate herbivore populations, in turn releasing plants from regulation by herbivory. Sea otters and kelp forests provided early empirical support for this hypothesis. The sea otter s role in structuring kelp forest communities was discovered by contrasting islands in the Aleutian archipelago at which the species was present in large numbers or absent, a serendipitous consequence of the Pacific maritime fur trade. Shallow reef habitats at islands with abundant sea otters had few sea urchins and well-developed kelp forests, whereas abundant sea urchins had destroyed the kelp forests at islands lacking sea otters (Fig. 5). The explanations tor this pattern is a straightforward consequence of what has since come to be known as a “trophic cascade.” That is, sea urchin populations are regulated by sea otter predation, in turn allowing the kelp forest to flourish in the absence of significant herbivory. When otters were removed.
Figure 5 Alternate community states in areas with (kelp forests) and without (sea urchin barrens) sea otters. Photographs were taken of reef habitats at about 10-m depths in the western Aleutian Islands. .
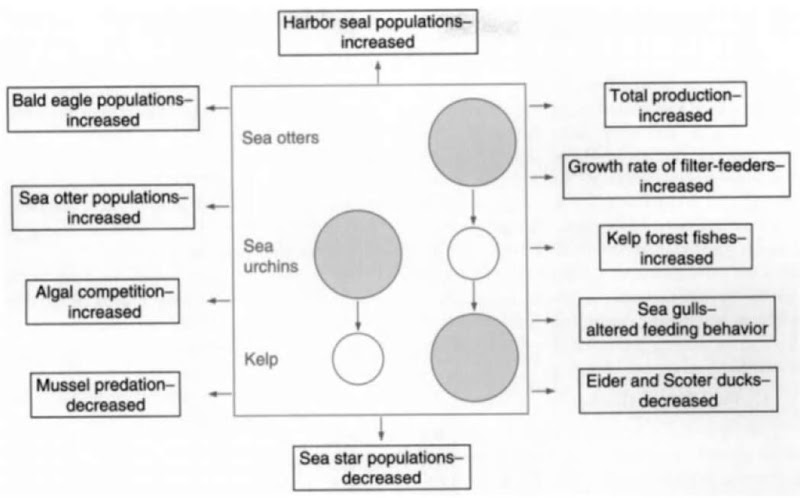
Numerous indirect effects of the sea otter-urchin-kelp trophic cascade are known or suspected (Fig. 6). Most of these relate to the role of kelp as habitat and a source of production for other species of consumers in coastal food webs. The kelps (order Laminariales) and other species of macroalgae are extremely productive, in large measure because none of the essential ingredients for photosynthesis (light, water, C02, and nutrients) are limiting in shallow coastal waters at high latitudes. Thus, systems with and without sea otters (and thus with and without well-developed kelp forests) vary substantially in total productivity. This fact was confirmed using the naturally occurring stable carbon isotopes to measure the relative pho-tosynthetic contributions of macro- vs microalgae across islands of the Aleutian archipelago with and without sea otters. Total production was estimated to be three to four times greater where sea otters were present. Further tests of the otter-productivity hypothesis were done by outplanting recently settled barnacles and mussels from a common source to intertidal and subtidal habitats at islands with and without sea otters. Growth rates were two to five times greater in otter/kelp-dominated systems.
The limiting effects of otters on sea urchins and other herbivorous invertebrates reduce the disturbance from herbivory, thus enhancing the strength of competitive interactions among kelp species. This effect has been demonstrated in the Aleutian Islands, Southeast Alaska, British Columbia, and central California. Further indirect effects on higher trophic level consumers result from increased production, altered habitat, or competition with otters for common food resources. The production effects are so strong that they almost certainly influence numerous co-occurring species. Bald eagle (Haliaetus leu-cocephalus) and harbor seal (Phoca vitulina) densities are substantially greater on islands of the Aleutian archipelago that also support abundant sea otter populations. Radically different diets and patterns of foraging behavior occur in glaucous winged gulls (Larus glaucescens) between sites with and without sea otters. Sea otters also appear to influence the population densities of benthic feeding sea ducks. Common eiders (Somateria mollissima), which feed mainly on sea urchins, mol-lusks, and other benthic invertebrates, occur at higher densities where otters are rare or absent than where they are abundant. Otters also influence predator-prey interactions between sea stars and mussels in the Aleutian archipelago. The biomass of sea stars at Attu Island declined by roughly two orders of magnitude following the spread of sea otters into Massacre Bay along the islands southeastern shore, in turn substantially increasing the survival rates of mussels and barnacles. This example is of particular interest because the influence of sea star predation on mussel beds (enhanced species diversity by the predation-induced reduction in competitive exclusion by the dominant mussels) is one of the most well-known and influential paradigms in ecology. The influence of sea otters on kelp beds even appears to elevate the environmental carrying capacity for otters themselves. Although sea otters limit the abundance of their benthic invertebrate prey, coastal fish populations are enhanced by increased production and the habitat provided by kelp forests. Besides elevating the equilibrium density, this interaction may stabilize sea otter populations because the fishes are less vulnerable than benthic invertebrates to population regulation by sea otter predation.
Figure 6 A schematic of trophic cascades in kelp forest systems with and without sea otters (central box). Known or suspected indirect effects of these interactions are represented at the peripheries.
The top-down influences of apex predators through trophic cascades have been incorporated into a conceptual model relating the strength of plant-herbivore interactions to trophic complexity (Fig. 7). The model predicts that plant-herbivore interactions should be strong in food chains with an even number of trophic levels (e.g., two, four, and so on) and relatively weak for odd-numbered food chains (e.g., one. three, five, and so on). The accumulated evidence from three decades of research on sea otters and kelp forests is highly consistent with that view.
2. Evolutionary Forces Strong species interactions maintained over sufficiently large scales of space and time should inevitably lead to selective responses in the interacting species. This expectation, coupled with predicted differences in the strength of plant-herbivore interactions between odd- vs even-numbered food webs, has led to a view that kelp forests in the North Pacific Ocean evolved in the absence of intense her-bivory. While the kelps have no fossil record, a variety of evidence indicates that they radiated in the North Pacific Ocean during the late Cenozoic in concert with sea otters, their recent ancestors, and other groups of benthic feeding marine mammals. The hypothesis that predators decoupled the coevolution of kelps and their herbivores was evaluated by contrasting various features of marine plants, invertebrate herbivores, and plant-herbivore interactions between North Pacific and Australasian kelp forests. Australasian kelp forests were chosen for this contrast because they lack predators of comparable effect to sea otters. Because kelp forest systems in the North Pacific and Australasia, respectively, evolved as odd-numbered (three) vs even-numbered (two) systems, the coevolution of plant defenses and herbivore resistance to those defenses were hypothesized to be strong in Australasia and weak in the North Pacific.
Marine algae were known to use secondary metabolites as their chief defense against herbivory. Therefore, the initial test of this evolutionary hypothesis was to compare the diversity and concentration of secondary metabolites between North Pacific and Australasian seaweeds. This comparison demonstrated that phlorotannins (the principal secondary metabolites in brown algae) were roughly an order of magnitude more concentrated in southern hemisphere kelps, in some cases approaching 20% dry weight. A second prediction of the hypothesis was that herbivore resistance to algal secondary metabolites is stronger in Australasian than in North Pacific species. This prediction was tested by extracting the metabolites from both North Pacific and Australasian seaweeds and measuring their strength of feeding deterrence to Pacific and Australasian herbivores. Regardless of their source, the strength of deterrence by phlorotannins was dramatically greater on North Pacific herbivores, thus suggesting that resistance to these compounds had evolved in southern hemisphere herbivores. This perspective and supporting data suggest that sea otters, their recent ancestors, and perhaps other predators have decoupled the coevolu-tion of plant-herbivore interactions in North Pacific kelp forests, thus explaining why North Pacific kelp forests were so vulnerable to destructive grazing following the demise of sea otters and other benthic feeding predators.
Figure 7 Fretwell’s (1987) model of alternating plant-herbivore interaction strength with increasing food chain length.
G. Population Biology
1. Genetics Sea otters provide unique opportunities for the study of marine mammal population and conservation genetics. The sea otter’s dependency on benthic/demersal habitats and limited diving capacity restrict its distribution to shallow coastal environments. Its small home ranges (generally <20 km of coast) probably restrict gene flow. Extensive human harvests reduced and fragmented sea otters into a small number of isolated colonies, from which all current populations are derived. Additionally, translocations from either one or two remnant populations have resulted in viable reestablished populations in Washington, British Columbia, and Southeast Alaska. Pleistocene glacial advances and retreats likely influenced genetic exchange. Isolation and extinctions caused by ice sheets extending over large coastal areas probably resulted during glacial maxima, whereas high levels of gene flow must have occurred as these areas were recolonized during periods of glacial retreat. Both morphological and genetic examinations of sea otters indicate some level of population structuring prior to 18th and 19th century fur harvests.
Sea otter skull size declines from Russia, across Alaska, and into California, and variation in skull morphology forms the basis for the three currently designated subspecies: Russia, E. I. lutris; Alaska, E. !. kemjoni, and California, E. !. nereis. Mitochondrial DNA (mtDNA) haplotype frequencies identify at least four distinct grouping of sea otters: California, Prince William Sound, the Kodiak/Aleutian/Commander Islands, and the Kuril Islands. The magnitude of difference in current mtDNA haplotype frequencies suggests at least some level of genetic differentiation among these groupings existed prior to the population declines and isolation that occurred between 1750 and 1900. The extant California population probably represents a monophyletic mtDNA lineage that contains two unique mtDNA haplotypes.
The extent to which long-term evolutionary processes and recent human exploitation have contributed to modern genetic differences among and within the subspecies of sea otters is unknown. The degree of difference in mtDNA haplotypes suggests that there has been little gene flow across the range of the species from California to the Kuril Islands of Russia. However, low levels of mtDNA sequence divergence in sea otters across their range also suggest that major phylogenetic breaks or long-term barriers to gene flow do not exist.
There is concern over the potential for loss of genetic diversity stemming from severe population bottlenecks experienced by sea otter populations over the past two centuries. However, theoretical analyses suggest that as little as 23% of the original genetic variation was lost from the California population because of this bottleneck effect. Subsequent empirical studies show that a high proportion of diversity was maintained during the period of recent isolation among populations, thus supporting this theoretical result.
Reintroduced and remnant sea otter populations provide the opportunity to study the effects of the duration and magnitude of bottlenecks on genetic diversity and population growth rates. In sea otters, mtDNA haplotype diversity is significantly correlated with both minimum population size and the number of years a population remained at that minimum size. No relationship was detected between genetic diversity and population growth rates, although translocated populations demonstrated significantly higher annual growth rates (\ = 1.18-1.24) than remnant populations (\ = 1.06-1.09). Genetic diversity is greater in translocated populations that were derived from two source populations compared with those derived from a single source population. Haplotype frequencies in populations with estimated founding sizes of 4 and 28 animals (Washington and British Columbia) differed from the source populations, probably signaling the effect of genetic drift. This interpretation is supported by the fact that haplotype frequencies from sea otters in Southeast Alaska, a translocated population with an estimated founding size of 150 individuals, did not differ from the source populations.
As sea otters continue to reoccupy former habitat, and currently isolated populations become contiguous, we may be afforded the opportunity to view the process of genetic exchange across the species’ range. Genetic differences currently observed among geographically isolated populations may diminish, reducing current levels of genetic population stnicture.
2. Demography As in all sexually reproducing species, sea otter populations are regulated by age- and sex-specific rates of reproduction and survival. These life history patterns in sea otters are more similar to those of the pinnipeds (with whom they share the ocean as a common environment) than they are to the other lutrines (with whom they share a more recent common ancestor). Perhaps the most remarkable life history feature of sea otters is that they almost invariably conceive and give birth to a single voung, a character shared with other marine mammals but not other lutrines (Fig. 8).
Female sea otters typically become sexually mature at 3 years, occasionally earlier. The reproductive cycle is normally 1 year, with roughly 6 months from conception to birth and another 6 months from birth to weaning. Substantial learning occurs during this latter period. Primiparous females usually fail to successfully wean their pups, probably because they have not yet learned to be good mothers. Adult females apparently enter estrus within several hours to a few days after mother/pup separation (either from weaning or death). The majority of preweaning deaths occur shortly after birth. Thus, in many areas there is a biannual peak in births, with the primary peak occurring in spring or early summer and the secondary peak (by females who failed in their previous cycle) in fall or early winter. Females continue to reproduce throughout life, with little or no evidence for either reproductive senescence or adjustments of fertility rate to environmental variation.
Figure 8 A female sea otter with her pup.
Population regulation in sea otters occurs largely or exclusively through variation in age- and sex-specific mortality rates. The high rates of population growth that have been observed in parts of Alaska, Canada, and Washington (17-20% per year, near the theoretical Rmax) could only be realized if the probability of mortality from birth to senescence were very’ low. As growing populations become limited by resource availability, the mortality rate in young otters increases greatly. Data from the Aleutian Islands and central California indicate that about half of the pups born fail to reach weaning age. The probability of mortality during the next 6 months, while as yet unmeasured, also seems to be relatively high. Mortality rates from about 1 year of age to physiological senescence (10-15 years) are low, even in food-limited populations. Thus, the principal mechanism of population regulation in sea otters appears to be starvation-induced mortality early in life.
Although the sex ratio at birth is about 50:50, the postweaning mortality rate is greater in males than in females, thus resulting in a female-biased adult sex ratio. The elevated male mortality probably is caused by aggressive interactions with adult males, thus forcing juvenile males to disperse into lower-quality habitats. These mortality patterns are reflected in the age and sex composition of beach-cast carcasses at locations where populations are at or near equilibrium density. In California, the age composition of beach-cast sea otter carcasses more closely resembles the age structure of living populations, thus indicating an elevated mortality rate of prime-age animals. Because of the generally similar age-specific birth rates across all sea otter populations, this elevated mortality in prime-age animals is responsible for a depressed growth rate in the California sea otter population.
H. Behavior
The behavioral ecology of sea otters strongly reflects life in the sea. Vigorous grooming is the species’ behavioral hallmark.
Sea otters probably spend more time and energy grooming their fur than any other mammal. This is accomplished by rubbing, rolling, blowing, and splashing. Grooming is necessary for cleaning and replenishing air to the under fur.
Sea otters are unusual among both carnivores and marine mammals in the generally small size of their home ranges, which typically includes no more than several miles of shoreline. Both males and females occasionally move longer distances for uncertain reasons. Extralimital sightings in central Baja California and near Wrangel Island in the Chuchi Sea demonstrate that, on occasion, individual sea otters move hundreds of miles.
Adult male sea otters maintain territories that in California average about 40 hectares. Adult females apparently move freely among these territories, but the territory holder aggressively excludes juvenile males. The precise function of male territoriality in sea otters is unclear, although ultimately territories serve to increase reproductive success. Adult males frequently harass females with large pups in an apparent effort to force separation, thus inducing the female to enter estrus and bear his offspring. Copulation occurs repeatedly during brief consorts, after which the males and females separate. A male grasps the female’s nose in his mouth and rolls vigorously on the surface of the ocean to achieve intromission. Distinctive nose scars in adult females often result from this behavior. In severe cases, trauma to the nose and facial region may result in death to the female. Some males seem especially prone to such brutality. Upon killing their mate, these males usually continue to copulate with the corpse, sometimes for days following her death. Adult male sea otters have been observed attempting to copulate with young harbor seals, in two such instances killing the seals during the process.
This sea otter’s polygynous mating system (and the resulting high male libido) likely evolved in response to their unusually high population density, thus promoting male competition for females as the limiting resource in sexual reproduction. Polygynous mating systems are typical of all otariids and some phocids in temperate latitude systems but apparently are rare or absent in other species of otters. As is true for other polygynous species, male sea otters provide no parental care.
I. Conservation and Management
1. Asia The status of sea otter populations throughout most of Asia is uncertain. Sea otters currently occupy all of their historic range in Russia and have been reported from northern Japan. However, present-day abundance is believed to be less than during the preharvest period, as areas of low density occur along the Kamchatka Peninsula and northern Japan. Sea otters recolonized Bering Island, in the Commander Islands, in about 1983. This population continued to increase until 1990, at which time it declined by about 40%. The population has remained stable since that time at about 3500 individuals. Systematic surveys of sea otter populations have not been done throughout most of Asia as recent governmental change has resulted in dramatic reductions in support of conservation and research programs.
2. Alaska Remnant and reintroduced sea otter populations experienced rapid and widespread recovery during most of the 20th century, probably due in large measure to the superabundant prey resources that developed in their absence. In some areas these growing populations eventually attained levels where food or other resources became limiting. These patterns of recovery and growth have been heralded as one of conservation’s great success stories. However, there are new concerns over the sea otter’s long-term welfare.
a. oil spills and other forms of marine pollution. The Exxon Valdez oil spill killed large numbers of sea otters—the minimum mortality estimate was 750 individuals. Moreover, in the 10 years since the Exxon Valdez spill, sea otter populations in oil-impacted areas have recovered at an annual rate of 3.3% per year, far below the growth rate observed in this same population prior to the spill. This had led some to conclude that chronic effects from the Exxon Valdez spill continue in Prince William Sound.
In the early 1990s, high levels of PCBs were discovered in sea otters from the Aleutian archipelago. These now appear to be associated with past military activities in the area. Although concentrations measured in sea otter tissues exceed those known to be harmful in mink, presently there is no other evidence that they are having detrimental effects on sea otters.
b. human harvests. Alaska natives are permitted to harvest sea otters for traditional purposes. Presently, most harvested animals are taken from Southeast Alaska and the Kodiak archipelago. There are no restrictions on take and large numbers are currently being removed from some populations. Sustainable harvest levels and take quotas need to be developed from life table data and the spatial ecology of individual otters. Quotas also should take into account the status and genetic structure of populations, and the availability of food and space.
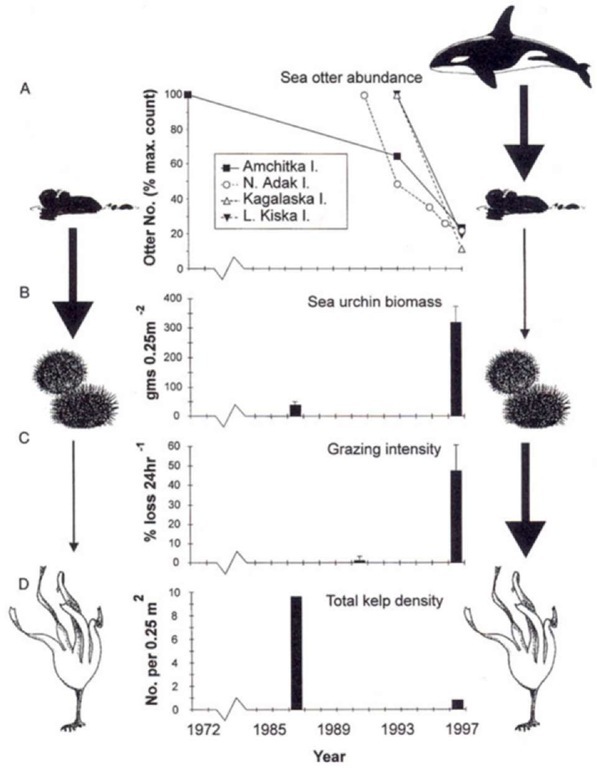
c. decline in central aleutians. During the 1990s the abundance of sea otters in the west-central Aleutian Islands has declined by about 80-90%. Available evidence indicates that these declines were caused by increased predation by killer whales. Killer whales may have switched from consuming pinnipeds to sea otters following the collapse of Steller sea lion and harbor seal populations in western Alaska during the late 1970s and 1980s. While the geographic extent of the sea otter declines appears to be large, additional surveys are needed to determine the range and magnitude of decline. As the result of these declines, sea urchin populations have increased, followed by a dramatic increase in the rate of herbivory and subsequent deforestation of the nearshore community (Fig. 9).
3. British Columbia The sea otter population in British Columbia continues to grow and expand its range. Conflicts with various shellfisheries are the primary concern of resource managers, whereas others believe that the population is still precariously small. Sea otter harvests have been proposed but at present are not permitted under either national or provincial law.
4. Washington/Oregon Like British Columbia, the Washington sea otter population continues to expand in numbers and range. From the relocation site near La Push on the outer coast, otters have spread around Cape Flattery eastward through the Straits of Juan de Fuca. These animals now occupy tribal waters of the Makah Indians and are rapidly depleting red urchin populations, which until recently supported a tribal fishery. Sea otters are presently absent from the coast of Oregon.
5. California California sea otters have long been the objects of debate and controversy. Fishery interests have maligned sea otters because they compete with humans for various commercial and recreational resources. Conservationists, however, have been concerned with the small size and slow growth rate of the California sea otter population. Increased trafficking of oil tankers and outer continental shelf oil development along the California coast have heightened concern that a spill might reduce or even extinguish the California sea otter population. For these reasons, the California sea otter population was listed as “Threatened” in 1977 under the U.S. Endangered Species Act. The U.S. Fish and Wildlife Service adopted a recovery plan for the California Sea Otter in 1981. Congress subsequently established Public Law 625 in an effort to both enhance the California sea otter population and protect shellfisheries. In 1987, under provisions of this law, sea otters were reintroduced to San Nicolas Island in southern California. The otter population at San Nicolas Island dwindled to about 15 individuals over the next several years, after which it remained relatively stable through the 1990s, despite the birth of at least 50 pups. Reasons for the growth failure of the population at San Nicolas Island are uncertain.
For most of the 20th century, the California sea otter maintained a slow but steady increase in numbers and range. There was a period of decline from the mid-1970s to the early 1980s, apparently resulting from increased losses in a coastal set net fishery. The population resumed growth almost immediately following the implementation of protective measures. The California sea otter population again began a gradual decline in the mid-1990s. Reasons for the current decline are uncertain. Reasonable possibilities include increased pollution and disease, incidental losses in fishing gear, and food resource limitation.
During this most recent period of numerical decline, the California sea otter’s range expanded southward, thus resulting in large numbers of otters moving south of Pt. Conception into the “no-otter zone” established by PL 625. Shellfishery interests are presently demanding that these otters be removed, whereas conservation groups are demanding that something be done to curtail the ongoing population decline. The U.S. Fish and Wildlife has yet to decide how they will respond to this unfortunate dilemma.
6. Mexico Except for occasional wanders from central and southern California, sea otters are currently absent from the waters of Baja California.
V. Concluding Remarks
After having been hunted to the brink of extinction, sea otters have recovered dramatically. While the status of other marine living otters is less certain, these animals appear better off than their freshwater-living counterparts, many of which are in a very bad way because of human exploitation and various kinds of habitat destruction. The relative well-being of marine otters is thought to result from habitat destruction being less problematic in the sea than it is on land. While that may be true, coastal marine environments increasingly are the recipients of terrestrial pollution and are being overexploited by numerous fisheries. Furthermore, scientists are beginning to understand that the coastal zone is linked in important and complex ways with both the open sea on its one side and land on the other. The fact that people live in disproportionately high densities along the land-sea margin is a direct threat to coastal marine ecosystems and many of the species that depend on these systems. All of this does not bode well for the future of marine living otters. Overall, sea otters are far more abundant today than they were 50 years ago. However, the recent and ongoing declines of otter populations in Alaska and California may be among the first signs from these animals that coastal oceans are in peril.
Figure 9 Changes in sea otter abundance over time at several islands in the Aleutian archipelago, and concurrent changes in sea urchin biomass, grazing intensity, and kelp density measured from kelp forests at Adak Island. Error bars indicate 1 SE. The proposed mechanisms of change are portrayed in the margins: the one on the left shows how the kelp forest ecosystem was organized before the sea otters decline and the one on die right shoivs how this ecosystem changed with the addition of killer whales, an apex predator. Heavy arrows represent strong trophic interactions; light arrows represent weak interactions.
In the process of recovery, expanding otter populations have come into conflict with fishery interests that developed during their absence. Similar conflicts surround other carnivores, but in few cases is the effect so apparent as it is with sea otters and shellfisheries. These conflicts strongly testify to the role apex predators often play in regulating their prey populations. Numerous other species and ecosystem-level processes are also influenced by the indirect effects of sea otter predation. Again, these examples have helped lead to the growing realization that apex predators often play complex but important roles in the maintenance of biodiversity, and thus that any affective strategy for biodiversity conservation must be sufficient to include viable populations of apex predators.