Vision and smell are senses that lose much of their usefulness in the watery medium where marine mammals live. As a result, hearing is generally more important in marine mammals than it is in land mammals. Hearing is a matter of life and death in odontocete cetaceans because they echolocate: they emit high-frequency sounds and determine the shape of their surroundings on the basis of the reflections of those sounds. Echolocation requires a sophisticated sound production organ (located in the nose) and sophisticated hearing. However, the physics of waterborne sound are very different than airborne sound (for a summary, see Denny, 1993). These two factors combined, the importance of well-developed hearing and the differences between sound in air and in water, have caused the organ of hearing to undergo a number of important changes. Some of these changes are well understood and occur across vertebrates that straddle the water-land boundary (Fay and Popper, 1985; Lombard and Hetherington, 1993). whereas others are the focus of intense investigations and controversies.
Sadly, sound is also important to marine mammals in another way. Dolphins may die if their middle ear is infected by nematodes because they are unable to detect prey. Loud sounds similarly may deprive whales and dolphins of their auditory abilities and may be a source of mortality.
I. Anatomy
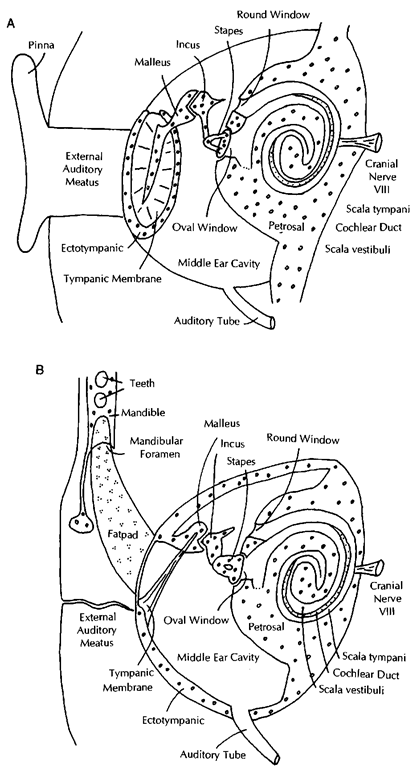
The organ of hearing of all mammals consists of three parts: the external ear, the middle ear, and the inner ear (Fig. 1A). Inland mammals, the external ear is basically an air-filled tube (the external auditory meatus) attached to a structure that funnels sound (the pinna). The tube ends medially at the tympanic membrane (eardrum), suspended by a bone called the ecto-tympanic. This tube fills with water when an animal enters the water. Medial to the tympanic membrane is the middle ear. It is an air-filled cavity, containing three small bones (ear ossicles) with two muscles attached to them. The bones are called malleus (hammer), incus (anvil), and stapes (stirrup) and form a chain contacting the tympanic membrane on one side (malleus) and the inner ear on the other (stapes). The middle ear cavity is air filled and is connected to the pharynx by means of the auditory tube (Eustachian tube), it does not fill with water when an animal is submerged. The inner ear is encased in a thick bone cover (the petrosal bone), it consists of a series of cavities in this bone that are filled with two kinds of fluids (perilymph and endolymph). For hearing, the most important of these cavities has the shape of a snail shell and is called the cochlea. It houses a long, hollow, rolled-up organ that mainly consists of three ducts that run the full extent of the cochlea. The scala vestibuli starts at the vestibule of the inner ear (where the oval window opens) and extends to the apex of the cochlea. There the scala vestibuli is connected to a second duct, the scala tympani. which extends from the apex to the base of the cochlea and ends at the round window. Both of these ducts are filled with perilymph. A third duct, the cochlear duct, is filled with endolymph and extends the length of the cochlea sandwiched between the two scalae. The floor of the cochlear duct is the basilar membrane in which a long row of neurons is implanted. The neurons of the organ of Corti are part of cranial nerve VIII (vestibulo-cochlear nerve) and pass auditory information to the brain.
Sea otters and polar bears have ears that are very similar to those of their land relatives. Pinnipeds and sirenians have ears that are, in gross features, similar to those of land mammals, although there are some important modifications (such as the absence of the pinna in phocids and sirenians). In cetaceans, the middle and inner ears still retain clearly recognizable anatomical features of land mammals, but the sound pathway through the external ear has been completely modified.
The cetacean external ear (Fig. IB) retains an external auditory meatus supported by cartilages, but the duct is narrow to the point where it is not patent and there is no pinna. Instead, the cetacean mandible is involved in sound reception. The posterior part of the odontocete mandible, behind the teeth, has a thin lateral wall (sometimes called the pan bone). This part of the mandible is medially concave and houses a large fat pad. This fat pad extends anteriorly into the mandibular foramen, which is enormous, and continues into the area ventral to the teeth. Posteriorly, this fat pad touches the lateral bony wall of the ectotympanic. This wall is extremely thin, and the attachment area for the tympanic membrane in cetaceans is reduced greatly. The ectotympanic has a joint (syndesmosis) with the petrosal (also called periotic), but the two bones combined have very limited connections to the remainder of the skull, unlike most mammals. In delphinids, this connection is limited to one small piece of cartilage; in all other odontocetes, the tympano-periotic has a larger connection; and in mysticetes.
Figure 1 Diagram of the ear in a generalized mammal (A) and a cetacean (B). Pinnipeds and sirenians have auditory systems similar to A.
The complex is integrated into the skull similar to land mammals. The entire tympano-periotic of odontocetes is surrounded by spongy tissue that is filled with air spaces.
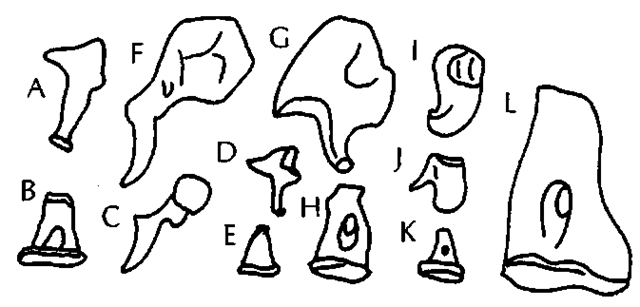
The tympanic membrane of odontocetes (often called tympanic ligament) is not the flat, more or less circular structure of land mammals. Instead, it is a strand of elastic tissue attaching to a small circular ectotympanic ring and then narrowing into an elastic tissue strap that attaches to the malleus (not unlike a folded-in umbrella). A similar structure occurs in mys-ticetes, but here an additional piece of the tympanic membrane forms a large, blunt protrusion into the external auditory meatus, often referred to as the glove finger. The ear ossicles of all cetaceans are highly pachyosteosclerotic and very different in shape from those of land mammals (Fig. 2). All cetaceans, except Kogia spp., have ossicles that are similar in overall form, and many of these morphological traits go back to the earliest Eocene cetaceans. In mysticetes, the stapes is much longer than in odontocetes. In Kogia spp., the malleus is fused to the ectotympanic in a unique shape. Sirenians and phocid pinnipeds (but not otariids and odobenids) also have pachyosteosclerotic ossicles, but they retain the shapes of the ossicles of their land relatives.
Middle ear muscles of cetaceans are reduced greatly. Suspended in the middle ear cavity is a plexus of veins and arteries collateral to the internal carotid artery. The internal ear of cetaceans is similar to that of land mammals, except that its proportions are different and that there are great differences among cetaceans (see Ketten, 1992).
The organ of balance in mammals consists mainly of three canals that run in the petrosal in a circular fashion. They are located immediately posterior to the cochlea and are also filled with perilymph and are innervated by cranial nerve VIII. Whereas the size of the cochlea scales closely to body size in mammals, the semicircular canals scale with body size in all mammals except cetaceans. In all modern cetaceans, the semicircular canals are much smaller than would be expected for their body size.
Figure 2 Auditory ossicles of marine mammals; all are left ossicles in similar views and to scale. (A and B) Ursus mar-itimus, incus, and stapes. (C-E) Eumetopias jubatus, malleus, incus, and stapes. (F-H) Lobodon carcinophaga, malleus, incus, and stapes. (I-K) Delphinus sp., malleus, incus, and stapes. (L) Dugong dugon, stapes. A-E represent more or less primitive morphologies for mammals, whereas all others are modified to various degrees.
II. Functional Morphology
Sound consists of waves of vibrations of the molecules that constitute air or water (in the case of marine mammals). The ear amplifies the sounds and translates them into neural impulses. Left and right ears together also gather directional information.
The pathway of sound in pinnipeds and sirenians is not significantly different from that of land mammals (Fig. 1A). Sound is funneled to the tympanic membrane, which starts oscillations of this membrane. These vibrations are transmitted to the manubrium of the malleus, which leans against the tympanic membrane. The vibrations are then passed along the chain of ossicles, eventually causing the foot plate of the stapes to pump in and out of the oval window. Ossicles function as an amplifier in two ways. First, sound energy that arrives at the tympanic membrane is concentrated on the much smaller area of the foot plate of the stapes. Second, the amplitude of the vibrations is enlarged by a lever-arm system: small vibrations of the long in-lever (the manubrium of the malleus) are transmitted to a much shorter out-lever (the crus breve of the incus). These two mechanisms result in higher pressures at the stapedial foot plate then at the tympanic membrane. Higher pressures are necessary to start vibrations in the dense fluid of the inner ear (perilymph). As such, the middle ear matches the acoustic properties of the air in the external auditory meatus to those of the perilyph of the inner ear and is technically often described as an impedance matcher.
The vibrations that are caused by the stapes set up standing waves in the fluids of the scala vestibuli. The standing waves are transmitted to the endolymph in the cochlear duct and these stimulate the hair cells on the basilar membrane. The hair cells fire electric impulses that are carried to the brain. Different frequencies are perceived by the stimulation of different sections of the basilar membrane: specific hair cells are receptors for specific frequencies. Low frequencies are perceived near the apex of the cochlea, whereas high frequencies are perceived near the base of the cochlea.
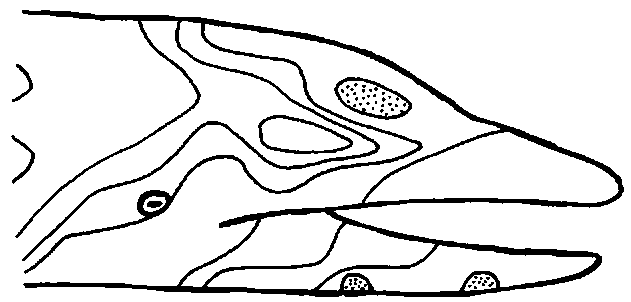
The inner ear of cetaceans functions in the same way as that of land mammals, but the external and middle ears are very different. The area that is most sound sensitive on a dolphin’s head is not the ear, but the skin over the lower jaw (Fig. 3). From here, sounds are transmitted through the bone of the mandible and through the fat pad to the bony wall of the middle ear. What happens here is controversial, but the best functional middle ear model for odontocetes has been proposed by Hemila et al. (1999). A model in which the entire ectotym-pano-ossicle complex rotates around an axis through the malleus correlates well with experimental data for hearing at low frequencies. A model in which four bony units (malleo-incus, stapes, ectotympanic, and periotic) are connected by springs that mainly allow translations predicts higher frequency data well.
The cetacean middle ear (and that of some pinnipeds as well) also contains a plexus of arteries and veins. It is possible that this plexus represents an adaptation for deep diving and that it can be inflated to reduce the airspace in the middle ear cavity, thus increasing its pressure to match ambient pressure.
The inner ear of cetaceans is structurally and functionally similar to that of land mammals, but differs in the details. Ket-ten (1992) distinguished two types of odontocete cochleas mainly on the basis of the shape and size of the basilar membrane. The differences are related to specific frequency ranges. Mysticetes have a different cochlea that is adapted to low-frequency sound reception.
Figure 3 One of the earliest determinations of sound sensitivity of the dolphin head. A hydrophone producing a sound of 65 kHz ivas pressed on different areas of this Stenella attenu-ata, resulting in this map of areas with similar sensitivities. The greatest sensitivity to sound (stippled areas) was on the lower jaw, with a lesser maximum on the forehead. The external auditory meatus (not shown, but located, posterior to the eye) does not represent a maximum of sound sensitivity.
The middle ear of pinnipeds and sirenians contains the same elements as in land mammals, but the ossicles of phocids and sirenians are greatly enlarged. This pachyosteosclerosis does not occur in odobenids and otariids.
III. Measures of Hearing
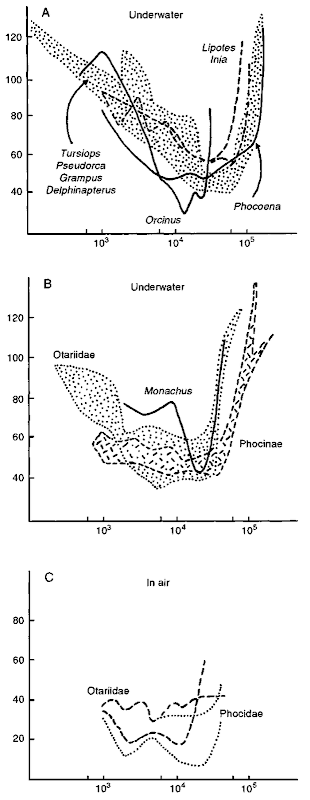
Hearing can be described by a number of quantifiable variables. Components of hearing include localizing ability (directional hearing), spatial resolution, and frequency discrimination (for a review, see Wartzok and Ketten, 1999). One of the most useful measures of hearing is the minimum audible intensity, which varies as a function of the frequency of the sound. The resulting plot is called an audiogram (Fig. 4). Sound intensities in these plots are usuallv indicated in decibels (dB), a relative and nonlinear measure that requires a reference intensity. To make matters even more complicated, it is customary to use different reference pressures for in-air and underwater measurements. Frequency is measured in hertz (Hz). The organ of hearing operates over five orders of magnitude, and frequency is therefore usually plotted on a logarithmic scale. Although straightforward to understand and display, determining audiograms and other measures of hearing is usually technically very hard, and results are partly dependent 011 factors outside of the control of the investigator (such as the motivation of the investigated animal if the audiogram is based on behavioral responses). For cetaceans, audiograms are only available for five families of odontocetes, four of which are represented by a single individual. Sample sizes are thus small, although some generalizations can be made.
Figure 4 Audiograms of cetaceans (A) and pinnipeds (B ) for waterborne sound; (C) for airborne sound. The X axis is frequency on a logarithmic scale in Hz. The Y axis represents pressure level at minimum audible sound (in dB. with 1 A as a reference for waterborne sound, and SPL for airborne sound). Hatched areas represent envelopes containing data for multiple specimens.
The delphinids Tursiops spp., Pseudorca crassidens, and Grampus griseus and the monodontid, Delphinapterus leucas show audiograms that have a fairly typical shape. For low frequencies (left side of Fig, 4A), minimum audible intensity decreases with increasing frequency. Auditory sensitivity peaks between 11,000 and 18.000 Hz (lowest part of the data envelope). and at higher frequencies, sensitivity is reduced. The peak in odontocete hearing is well above the frequency of best hearing in humans (2000 Hz, but variable with age) as a result of the use of high frequencies in echolocation. Optimal hearing in Phocoena spp. and Orcinus orca occurs at frequencies that are lower than for the delphinoids mentioned earlier. For Phocoena, the lower optimal frequency has been related to its particular cochlear shape (Ketten, 1992). Orcinus is a delphinid of large body size, and. in general, ears of animals with larger body sizes are tuned to lower frequencies, which is related to their longer cochlear duct (the size of the cochlea scales with body weight). The lower optimal frequency in Orcinus can also be understood in another way. The size of objects to be discerned poses limits on the echolocation frequencies that can be used in such a way that, to discern smaller objects, higher echolocation frequencies need to be used. Orcinus hunts larger prey than other delphinids and hence can use lower frequency signals. The freshwater dolphins Inia geoffrensis and Lipotes vexillifer have overall curves that are similar to those of the generalized delphinids but are less sensitive than in the generalized group. Several explanations are possible; one of them holds that these species hunt in small-scale river environments, where only prey close to the predator can be pursued. In open sea settings, prey that is further away can be pursued successfully. Echolocation signals and echos attenuate with distance, and hence a closer object can be discerned with a receiver that is less sensitive.
Underwater audiograms differ among different pinnipeds (Fig. 4B). Significantly, phocines (Phoca spp.. Pusa spp., and Pagophilus groenlandicus) consistently show better high-frequency sensitivity than otariids. The only monachine (Monachus spp.) for which an audiogram is available lacks the high-frequency sensitivity of the other phocids and is similar to the otariids in this respect. The differences between phocines and otariines do not occur in in-air audiograms (Fig. 4C), suggesting that pinnipeds, means of sound transmission differ in air and in water. The curious bimodal sensitivity peak in two individuals also remains to be explained and may be the result of the compromise that the pinniped ear represents, a compromise between hearing in air and in water.