The family Eschrichtiidae includes a single known genus and species, the gray whale, which now is found only in the North Pacific and Pacific Arctic Oceans (though it once lived in the North Atlantic until the 17th or early 18th centuiy). Grays are by far the most coastal of all the great whales, and inhabit primarily inshore or shallow, offshore continental-shelf waters. They tend to be nomadic, highly migratory, and are tolerant of climatic extremes. Each year, they make the longest migration of any whale (up to 15,000-20,000 km round trip) largely without feeding, traveling along nearshore routes between a summer feeding zone of high productivity in Arctic or subarctic waters and a winter breeding zone in temperate or subtropical southern waters. Unlike other mysticetes, the gray is primarily a bottom feeder and influences the topography of the seabed in the Arctic (from sucking its prey out of the sediments). It is the only whale to bear its young in warm, shallow, coastal areas and lagoons.
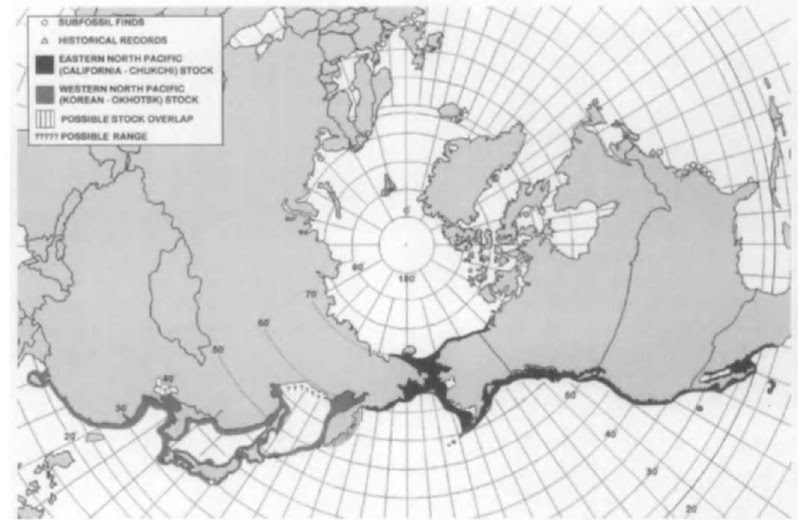
There are two populations. The western North Pacific population (or Korean-Okhotsk) migrates along the coast of Asia. It was hunted to the verge of extirpation and is extremely rare. Another much larger, eastern North Pacific population (or California-Chukchi) migrates along the coast of North America and eastern Siberia (Fig. 1). It too was severely overexploited in the latter half of the 19th and early 20th centuries, but, following protection from commercial whaling, has increased to about 26,600 (in 1999). The resilient eastern North Pacific gray whale is the only cetacean population that, following severe depletion, has sufficiently recovered under protection from commercial whaling to be removed from the list of endangered species. The western Pacific population, however, remains listed as critically endangered.
An active but gentle species, as long as they are not molested, the gray whale had a reputation for ferocity among the old whalers, who dubbed it “devilfish” for its habit of crashing into and staving in boats when harpooned or in defense of its young. Despite it being the trickiest and most dangerous prey, early whalemen developed a special affection for grays and found them to be the most interesting and intelligent of all the great whales. Grays seemed to learn quickly the dangers from whirling and performed a remarkable array of evasive maneuvers. They were admired for their fierce protection of their young and habit of giving assistance or “standing by” an injured companion, often reaching self-sacrificing measures. When attacked, they showed a power of resistance and tenacity of life that distinguished them from all other cetaceans. Today, many people have come to value gray whales more highly as a living resource than as one to slaughter, and they have become a whale-watching phenomenon. Their coastal habits make them the most accessible of all the mysticetes and they can be seen most easily, often from shore. Gray whales are unusually sportive; breaching, spy-hopping, lobtailing, and mating extravaganzas are essential elements of their migratory and breeding-grounds repertoires. Their willingness to allow whale watchers to stroke them is an added attraction, and grays are now known as “friendly” whales.
I. Systematics
A. Evolutionary History and Classification
No fossils of a direct gray whale ancestor have been found. The family Eschrichtiidae is known only from the Recent and from a single Pleistocene specimen about 50,000-120,000 years old found in California, and a less certain one from Alaska. A long-held theory proposed that gray whales could have evolved from the Cetotheridae, an extinct family of whales dating back some 38 million years, and could be their closest living relatives. Due to the lack of any fossil remains linking the modem gray whale to the far more ancient cetothere, some challenge that view and are unwilling to link them to any of the known early whales. A highly distinctive species, the gray whale has been placed in its own family: Eschrichtiidae (Ellerman and Moirrison-Scott, 1951) ( = Rhachianectidae, Weber, 1904).
Most experts have considered the gray whale to be more closely related to the rorquals (Balaenopteridae) than to the right whales (Balaenidae). Others have given it an intermediate position between the two. However, for the four modern families oi baleen whales commonly recognized (Balaenopteridae: rorquals, or fin whales; Balaenidae: bowhead and right whales; Eschrichtiidae: the gray whale; and Neobalaenidae: the pygmy right whale), the pattern of phylogenetic relationships at the base of baleen whale divergence is unresolved. With respect to gray whales, analyses of their position within the Mvsticeti conflict. Molecular studies position gray whales within the balaenopterids, while analyses based on morphology and including fossil and extant taxa differ in suggesting grays are linked with the balaenids and the pvgmy right whale. Moreover, some biologists place the gray whale as a subfamily of Balaenopteridae.
Figure 1 Known distribution, historic and current, of the gray whale. The eastern North Pacific population (black) has recovered from depletion. The western North Pacific population (gray) remains critically endangered. The Atlantic gray whale is extinct and is known from subfossil finds (circles).
B. Names
The gray whale has many English names, first applied by 19th century whalers. Scrag was used by old whalers on the Pacific coast of North America because they identified it with a whale called a scrag that was taken in the Atlantic Ocean in the 17th and 18th centuries. Devil fish and hard head were derived from the often violent reaction of the grays that commonly smashed boats with their heads and flukes when harpooned. Mud digger and mussel digger referred to the bottom feeding of the whales. Gray and gray back characterized its color. Okhotsk and Korean denoted the western population’s feeding and presumed breeding grounds, and Chukchi and California, the feeding and breeding grounds for the eastern population (also the whaling grounds).
As for its scientific name, the generic name Eschrichtius (Gray, 1865) was given to honor a 19th century Danish zoologist, Daniel Eschricht; and the specific name robustus (LiUjeborg, 1861) is from the Latin for “oaken” or “strong.” The gray whale first became known to science not through observations of living animals but through the discovery of subfossil skeletal remains from Europe where it had long been extinct. Conspecificity cannot be proven by purely anatomical data, but the skeleton of the gray is distinctive and no anatomical difference has been found between extinct Atlantic and extant Pacific populations (or between the eastern and western populations of the Pacific) that would justify separating them on the basis of species, or even subspecies. Thus, the odd situation exists where the remains from the extinct Atlantic population serve as the type specimen for the Pacific Ocean (=Eschrichtius gibbosus Erxleben, 1777).
II. Description
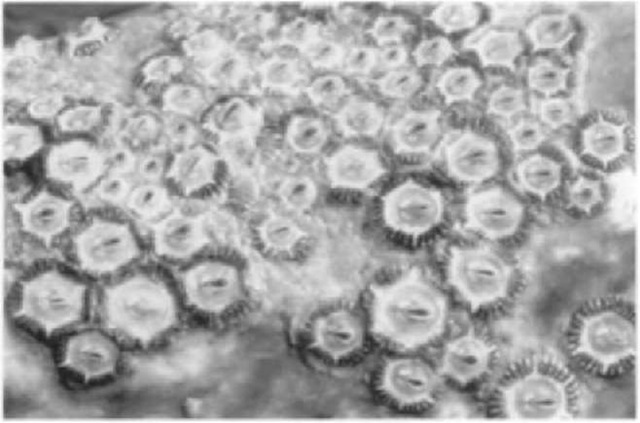
The gray whale is a robust, slow-moving whale with a flexible body, more slender than the right whales and more stocky than most rorquals (Fig. 2). This species is readily identified by the mottled gray color of the skin with numerous lighter patches scattered all over the body (although color may vary from gray-brown to slate-black). Grays have more external parasites and epizoites than any other cetacean. The barnacle, Cryptolepas rhachianecti, thought to be host specific, has been found on beluga whales (Delphinapferus leucas). As larvae, barnacles are free swimming but soon settle onto calves and adults alike.
Figure 2 The narrow head of the gray whale is usually covered with patches of barnacles and whale lice (top left). The blow is heart-shaped and 3^1 m high (top right). Instead of a dorsal fin, grays have a low hump followed by a series of bumps (bottom left). The flukes are over 3 m wide, frequently bear scars from the teeth of killer whales, and are often lifted before a deep dive (bottom right).
Figure 3 Dense clusters of barnacles surrounded by whale lice develop shortly after birth. Barnacles leave white scars on the ivhales skin, which slowly repigments over time.
Eventually forming large colonies that are deeply embedded in the skin. Grays also host three species of whale lice (they are cyamids, not insects) that feed on skin and damaged tissue: Cyamis scammoni and Cyamus kessleri occur only on grays, whereas Cyamus ceti also lives on other whales (Fig. 3). The lice cling by the thousands in areas of reduced water flow, such as around barnacle clusters, blowholes, and folds of skin, and swarm into wounds. In the breeding lagoons, schools of topsmelt (Atherinops affinis) symbiotically clean lice and sloughing skin from the whales. Much of the whale s mottled appearance comes from the parasites or scars from previous infestations and abrasions. By photographing the skin pigmentation patterns on the backs and sides, it is possible to identify individual animals, which is important to the study of gray whales.
The gray whale’s relatively short, narrow head is triangular (in top view) and moderately curved downward (in side view) (Fig. 4). It is encrusted with patches of barnacles and associated whale lice, particularly on top. Widely spaced bristles sprout from small dimples on the upper and lower jaw (no other whale has so many hairs); these short bristles, linked with sensory cells, are extra noticeable on calves. The skull comprises only about 20% of the total skeletal length. A unique feature is the presence of paired occipital tuberosities on the posterior part of the skull. Small eyes, with eyelids, are located just behind the corners of the mouth. Directly above them, on top of the head, is a pair of blowholes (nostrils). Barely visible, the ear opening is a tiny hole just behind the eye. The narrow upper jaw has 130 to 180 baleen plates hanging down on each side, separated in the front of the snout. A gray’s baleen is the shortest (5-40 cm), thickest, and coarsest of all mysticetes and is cream-white to pale yellow. The lower jaw is broad, with a keel-like protuberance in front, and slightly arched. On the throat there are two to seven (commonly three) short, deep creases that stretch open and allow the mouth to expand a little during feeding, but they do not extend beyond the throat region and are insignificant compared with the many long ventral grooves found in balaenopterids.
Gray whales lack a dorsal fin but have a low hump followed by a series of 8-14 small bumps (knuckles) along the top of the tail stock. The ventral part of the body is smooth, without any longitudinal grooves. The paddle-shaped flippers are up to 200 cm long. Tail flukes are over 3 m wide on adults, with smooth trailing edges and a deep median notch. The flippers and flukes are often marred with tooth scars from killer whales (Orcinus orca). Unique to this species is a cyst-like structure (10-25 cm in diameter) beneath a swelling on the ventral surface of the tail stock, which may be similar to sebaceous glands of land mammals, or function as a track-laying scent gland, although its exact function is unknown. Grays, which survive in extremely cold water for about half of the year during the feeding season, are insulated with a layer of blubber averaging 15 cm thick beneath the skin and can tolerate a great drop in their skin surface temperature to only a degree or so above that of the surrounding water.
Figure 4 Gray whales commonly spyhop, lifting the head vertically above the water. The head is narrotv and triangular when viewed from the top (left), and they have from two to seven short creases on the throat (right), rather than the long, ventral throat grooves found in balaenopterids.
Newborn grays (calves) average 4.6 to 4.9 m long and weigh about 920 kg. The sex ratio is parity at all ages. They reach puberty at anywhere from 6 to 12 years of age (average is 8), at a mean length of 11.7 ni in females (called cows) and 11.1 m in males (called bulls). Adults weigh 16,000 to 45,000 kg and stop growing at about 40 years, when the average female is 14.1 m long and the average male is 13.0 m. The largest female recorded was 15 m, and the largest male 14.6 m long. Although adult females are slightly bigger than males, there is no significant difference in their appearance (the distance from the genital slit to the anus is wider in males). The maximum, as well as average, life span is unknown (age is calculated from growth layers in the waxy ear plugs that fill the auditory canal). One large female was estimated to have been 75-80 years old when she was killed and she was pregnant.
III. Ecology and Behavior
A. Social Organization
The gray whale is not a highly social species. Individuals may associate with many conspecifics, but they do not appear to form stable pairs or groups and come together for only part of the year during migration and on the winter breeding range. The only persistent social bond known is between a mother and a calf, which disappears at weaning. Now and then, short-term associations lasting several days or weeks are reported, but their significance is still a mystery to us. Very little research into the social organization of the gray whale has been done. It is possible that they communicate even over large distances, sending and receiving acoustic signals. No territoriality, dominance, or overt aggression toward conspecifics has been reported.
On the summer feeding grounds, grays are usually widely spaced, solitary (commonly pregnant females), or in pairs, and less often in small groups of 3-5, although many may be in proximity in the patchily distributed food-rich areas. Larger aggregations in tens or even hundreds can occur in a particularly rich feeding area but are likely related to a mutually available mass of food rather than to social cohesion or interaction (these aggregations fluctuate constantly). Occasionally, some grays stop feeding to form groups of 30-40 or 100-400 animals that engage in bouts of social activity (lasting 1^1 days) reminiscent of courting and mating; however, their function is unknown. During migration, singles, pairs, and trios are most common but grays sometimes form transient groups of up to 16 individuals.
On the winter breeding grounds, large aggregations of mothers with young and courting/mating whales are common, but are in constant flux (1000 or more will crowd into the largest breeding lagoon). Initially, mothers with neonates have little interaction with other mothers and calves, although many are concentrated in the nursery areas of the breeding grounds. When calves are 2-3 months old, however, they often form highly interactive social groups. In these encounters, mothers and young cavort en masse, rolling about on top of each other. rubbing and touching from head to flukes, and often emitting huge bursts of underwater air bubbles. Groups last from a few minutes to over 3 hr and involve up to 40 individuals at a time, with many others coining and going, and may play a role in the social development of the calf.
Overall, there is a low degree of cooperation among gray whales, except limited examples of joint defense against killer whale attacks and assistance or support behavior, mainly for the aid of the young and especially in the calving areas. This is evidenced by adults coming to the aid of a mother whose calf is in trouble. Standing by (whales in a pair or group assisting, supporting, or staying with an injured companion) also occurs occasionally among adults in times of distress.
B. Feeding
Gray whales do almost all of their feeding during summer and fall when they are in higher latitudes, where they forage on the ocean floor in shallow waters over continental shelves (4-120 m deep). They are adapted to exploit the tremendous seasonal abundance of food that results as the Arctic pack ice (sea ice that is unattached to land) retreats in spring, exposing the sea to the polar summers continuous daylight, which triggers an enormous bloom of microorganisms in the water down to the sea floor. Unlike other baleen whales, the gray is mainly a bottom feeder and sucks small invertebrates and crustaceans out of the sand and mud. Their distribution in the feeding grounds coincides with the concentrations of these bottom-dwelling prey. As the summer advances and the edge of the pack ice recedes and uncovers more of the feeding grounds, the whales move. They feed heavily from about May through October, gaining enough stores of fat to sustain them during fasting or greatly reduced intake of food during the rest of the year, when the polar feeding grounds are ice covered and they migrate south to warm winter breeding grounds.
During about 5 months of intensive feeding in Arctic waters, an adult will consume roughly 170,000 kg of food. By the time the grays return to the feeding grounds (5 to 6 months later) they will have lost up to 30% of their body weight and must single-mindedly forage to replenish their fat reserves. The highest energy costs during migration are incurred by pregnant or lactating cows. For cows, the cost of reproduction includes the energetic requirements for gestation (producing a calf) and lactation (nursing young until weaning), which is far greater. During summer and fall, pregnant cows put on 25-30% more weight than other gray whales (exclusive of fetus).
An extraordinary aspect of the gray whale’s feeding ecology is its apparent dietary flexibility. Over 80 species of prey have been identified, reflecting its opportunistic approach to foraging. On the summer feeding grounds, grays primarily consume benthic gammaridean amphipods (shrimp-like crustaceans that live on or buried in the sediment). Amphipods from four families account for about 90% of the food, but depending on the feeding area, 1 of 7 species is usually dominant. Four are from the family Ampeliscidae (Ampelisca macro-cephala, A. eschrichti, Bijblis gaimardi, Haploops sp.). They are tube builders that live in dense colonies or “tube mats” in the upper few centimeters of sea floor sediments. Overall, the am-phipod A. macrocephala (up to 33 mm long) is probably the most commonly taken species (and occurs in concentrations as high as 23,780/m2 in the Chirikov Basin in the Bering Sea). The other three species are from separate families: Haustori-idae (Pontoporia femorata), Lysianassidae (Anom/x nugax), and Atylidae (Atylus bruggeni), which are mobile scavenging am-phipods that rove freely over the seafloor in search of prey. In some areas, polychaete tube worms (Travisia forbesi) are their main food. Planktonic prey items eaten in the peripheral feeding areas south of the main feeding grounds occur in swarms or schools and include mysids, crab larvae, red crab, mobile amphipods, herring eggs and larvae, squid, megalops, and bait fish. Some plant material also occurs in their stomachs.
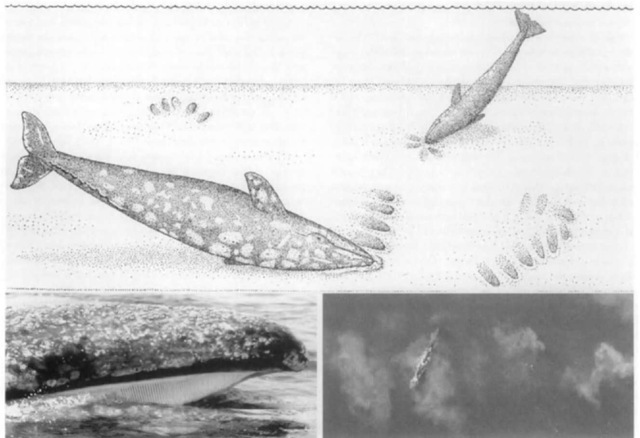
To bottom feed, grays roll to one side, bringing the head parallel with the seabed, sweep the side of the mouth close over the bottom a few centimeters above it, and open the jaws slightly to suck sediment containing prey into the mouth (which has flexible lips) (Fig. 5). Water, sand, and mud are strained through the comb-like baleen, leaving the food trapped on its inner margin. The suction might be created by retracting the large, strongly muscled tongue (weighing 1400 kg). The grays move slowly along the bottom, sucking up infauna in pulses, and surface with clouds of sediment (called mud plumes) streaming from the mouth. Mud plumes mark the meandering path of the feeding whales. Seabirds feed on prey brought to the surface in the plumes.
Grays impact their feeding grounds more than any other cetacean. Bottom feeding leaves mouth-sized depressions or “feeding pits” in the sea floor, from which the top layers of sediment are removed. Foraging is a major source of physical disturbance to the benthic community and plays an important role in the rate of turnover of the epibenthos. In some areas of the Arctic, over 40% of the seafloor is pock-marked with feeding pits. It is thought that by clearing space in the bottom, whales open areas for recolonization, succession, and maturing of the prey community, thus promoting the growth and diversity of life on the seafloor. Periods of nonuse are presumed to correspond to rapid recover)’ of the habitat. However, if the resource is overutilized and the area is stripped, it could be a one-way street leading to the permanent loss of amphipod communities and changing feeding patterns. In this way, gray whales are an integral part of the coastal community and participate in a dynamic feedback loop, termed “niche construction,” whereby their feeding activities function to shape their ecological niche through alteration of the benthos.
In addition to bottom feeding, grays also occasionally feed by surface skimming and engulfing planktonic prey out of the water column. Zooplankton are only known to be utilized outside of the principle feeding grounds, in peripheral feeding areas throughout the migratory range. Instead of traveling the entire distance to the feeding grounds, some whales spend the summer feeding along the coast in other parts of their range. Also, whales destined for the summer grounds sometimes stop to feed periodically on the way if the opportunity arises. The importance of peripheral feeding areas is unclear. With three modes of feeding (benthic suction, engulfing, and skimming) the gray has perhaps a greater range of foraging techniques than any other of the great whales.
Figure 5 A bottom feeding gray whale swims on one side to suck prey from the seafloor, creating mouth-sized depressions or feeding pits (top). The cream-white baleen plates are the coarsest, shortest, and fewest of any mysticete (bottom left). Sieving prey-laden sediments through the baleen creates billoiving mud plumes (bottom right).
C. Reproduction
Gray whales are thought to have a promiscuous mating system: males and females do not form long-term pair associations and both sexes may copulate with several partners during the same breeding season. Because multiple inseminations can occur, it is proposed that sperm competition may be taking place in gray whale fertilization (sperm from two or more males compete to fertilize the ovum within a female). Adult males have relatively large testes weight (averaging 38 kg in mating season) to body weight ratios and presumably produce large quantities of sperm. In this mating strategy, copulating males attempt to dilute or displace the sperm of other males to increase the likelihood of being the male to fertilize the female. Their fibro-elastic penis reaches 170 cm in length and is erected by muscle fibers and not vasodilation.
Reproduction in gray whales is strongly seasonal. The female reproductive cycle lasts 2 years and consists of the onset of estrus (the period of sexual receptivity), ovulation, conception, gestation, lactation, and an anestrous period. Most females bear young in alternate years, although some may rest 2 or more years between calves. In general, each year one-third to one-half of the adult females are birthing (they are not receptive to bulls after calving) and the remainder are mating, with a reversal of roles each successive year. Cows continue to breed at an advanced age. Bulls mate annually. They have a peak of spermatogenetic activity in late autumn or early winter, correlating with the time females come into estrus. Some sexual behavior on the feeding grounds and among males occurs that apparently serves nonreproductive social purposes.
Lengthy courting (precopulatory) behavior is part of the mating process, evidently requiring sufficient physical contact by the bulls to arouse the cows, but detailed information on the constituents of courtship is not yet available. Copulation occurs belly to belly. Pairs or trios of whales sometimes court and mate quite gently together. More often (or perhaps just more readily visible), there is a high level of activity, marked by whales rolling, touching, splashing, and cavorting energetically, at which times bulls with extended penises can be seen (Fig. 6). While some nudging and pushing may take place to get close to a cow, bulls do not appear to fight to keep others away. Bulls outnumber available cows by as much as two to one, leading to the belief of a menage a trois mating group by early naturalists. In fact, although trios are common, so are pairs and groups of various sizes that can blossom into a giant free for all involving as many as 20 consorting adults at a time. The large groups constantly fluctuate, with some participants departing while others join in as if stimulated by die sexual activity of the initial core group.
Figure 6 Gray whales mate with multiple partners, often in large, energetic courting groups (top). Newborn calves have nwre uniformly dark skin and are supported on their mothers’ backs for their first few breaths (bottom).
Conception occurs primarily in late November and December while the whales are migrating south from the feeding grounds, but some do not conceive until in the winter assembly area, or even on the northward spring migration. Length of gestation is disputed, but is generally thought to last 11 to 13 months, which means that newly pregnant females do not give birth until they have completed the following year’s southward fall migration. The birth season lasts from about late December to early March (median birth date: January 27), when most near-term cows are in or near the calving grounds, although some calves are born during the migration from California south. Cows bear a single calf, unattended, and provide sole parental care. Reports of births cite head-first deliveries, with die cow supporting her calf at the surface for the first few breaths of air. Initially, its movements are uncoordinated, but swimming soon steadies (Fig. 6).
A mother’s bond with her calf is especially close. She displays an unusual degree of affection, often gently stroking it with her flippers. Mothers are highly protective and will fight fiercely to defend their young from danger. While in the lagoons and on migration, calves stay close to and almost touching their mothers. They drink about 189 liters of rich milk (about 53% fat and 6% protein) each day and grow rapidly, reaching 8.7 m when weaned. The calf remains dependent on its mother until it is weaned ill the summer feeding area, at about 7-8 months of age, and perhaps 1 or 2 months longer, when they have solid food in their stomachs but remain with their mother. It is thought that calves begin to forage during the latter stages of nursing and thus may gain some experience while still with the mother. After the calves are weaned, around August, cows are anestrous for 3 to 4 months. Then in November to December a new mating period begins.
D. Sensory Perception
1. Acoustics Once reported to be almost silent, it is now known that gray whales are soniferous both dav and night. They create a variety of phonations that sound like rasps, croaks, snorts, moans, groans, grunts, pops, roars, quick series of clicks, belches, and metallic knocks and bongs. These low-frequency broadband signals range from about 100 Hz to 4 kHz, but may go up to 12 kHz. The most prevalent sounds for whales feeding in the Arctic and those in the breeding lagoons are pulsive signals, usually emitted in bursts, that sound like a series of metallic knocks (broadband pulses, from about 100 Hz to 2 kHz). Tonal moans are the most common phonation from migrating whales. Some behaviors may also serve an acoustic function. Grays expel huge bursts of air bubbles underwater (explosive exhalations). These emissions are often released in profusion from the blowholes in social settings. Occasionally, large quantities of air are released from the sides of the mouth as the whale swims by, producing a spectacular display of bubbles. The functions are still obscure, but the joint effect of the acoustic and visual components could create a potent short-range communication signal. Other behaviors that may have an acoustic function include percussive jaw claps, head slaps, back-slaps, breaching, flipper slapping, and lobtailing.
Gray whales are not known to echolocate by means of high-frequency click trains as odontocetes do. However, some low-frequency click-like sounds resembling echolocation (which enables a whale to detect objects by listening to the reflected echo of its own sound pulses) have been recorded. These sounds are very tentatively proposed as evidence for primitive echolocation aptitudes that may serve a long-distance function limited to large targets (such as whales) or to detecting broad topographical or oceanic features useful for orientation and navigation. The theorv of echolocation in gray whales, however, is as yet unsubstantiated.
Whalers have long stood in awe of the gray s sensitivity to sound. Even the water disturbance by an oar may put a whale to flight. The relatively low upper limit of the frequency range of their vocalizations suggests that tliev mav hear well into the low sonic or infrasonic regions (below the range of human hearing, frequencies lower than 18 Hz). The use of mostly low-frequency sounds is thought to be an adaptive strategy whereby grav whales circumvent the high levels of natural background noise prevalent in their coastal environment by producing sounds that are generally at frequencies below it. Unfortunately. much of the man-made noise in the ocean also occurs in the lower frequency range and has a high level of output, which could interfere with or mask the grav whale’s sounds or possibly damage their hearing. Grav whales appear to try to get around some man-made noise by increasing their call types, calling rates, and the loudness of calls to enhance signal transmission and reception.
2. Other Senson Perception Gray whales can see moderately well both in air and water, but color vision is probably weak. The position of the eyes suggests that they have stereoscopic vision forward and downward permitting efficient estimation of distance. The eyes are adapted for heightened sensitivity to dim light and for improving contrast and resolution underwater. Grays have retained some sense of smell but are microsinatic at best. In water, the nares are almost always closed (but whales may smell the air as they breathe). The sense of touch is well developed. Some taste buds occur at the back of the tongue, and the possibility of chemoreception through taste has been conjectured.
E. Sleep
It is not known if gray whales sleep. Whales on migration have not been observed to stop to rest for long periods of time. One exception is mothers and calves, which stop to nurse and rest during the north migration. In polar regions during summer when daylight is continuous, most gray whales remain active continually, usually foraging or moving between feeding areas. although occasionally a few resting animals are seen. It is speculated that grays, like some delphinids. may rest one hemisphere of the brain at a time (presumably essential to a voluntary breather). In the breeding grounds, there are more obvious indications that grays sleep, particularly near-term pregnant females and those with neonates. They rest, barely awash, floating at or just beneath the surface, with head and flukes hanging down, for up to an hour, and raise the head to breathe periodically in a slow rhvthinic pattern.
F. Swimming, Breathing, and Diving
Overall, gray whales are relatively slow but steady swimmers on migration, although speeds vary from the beginning to the end of the route and there are periods of wandering, resting, milling, feeding, and breeding activity in addition to directed travel along the way. They make the southward trip from the feeding to the breeding grounds in an average of 55 days, swimming at about 7-9 km/hr. and cover a distance of about 144-185 kin/day. On the north migration, grays move at a slower speed, averaging 4.5 km/hr (88-127 km/day), and may socialize and feed more, which effectively slows their diurnal rate of migration. Mothers and calves travel up to 96 km/day. Speed of directed travel is about the same as that of other whales, but mothers and calves pause to rest and nurse along the migration. When pursued, grays may reach about 13 km/hr but can only maintain this pace for a few hours. Speed under duress can surge to 16 km/hr, at least for short bursts (avoiding predators). Interestingly, gray whales are very efficient swimmers. They travel mostly at speeds that minimize their energy expenditure and maximize their range, and swim at depths that minimize total drag, important factors in successfully covering the long migratory distances thev travel.
Gray whales usually are not exceptionally long or deep divers. The pattern of breathing between dives can van’ greatly for different activities, with grays averaging only 3% of the time at the surface. When migrating, whales typically remain submerged during trav eling-dives for 3 to 5 min during which they mav travel 300 m. Thev surface to blow three to five times at intervals of 15 to 30 sec during a series of short, shallow, surface dives showing onlv a small portion of the back. The bushy spout is 3 to 4 m high (Fig. 2). Following the terminal blow in a series, traveling whales typically lift their flukes into the air (fluke up) to begin the next traveling dive. During prolonged dives, they may remain submerged 7 to 10 min (or longer) and travel 500 m or more before resurfacing to breathe. Usually, the longer the dive, the greater the number of blows, as the need to reoxygenate the system is greater. Their maximum known dive depth is 170 m.
Breathing and diving behavior on the feeding and breeding grounds is more variable than on migration. When foraging on summer feeding grounds in shallow coastal waters of 50-60 m, grays dive to the bottom by submerging almost vertically and lifting their flukes above water, and stay under for 5 to 8 min while swimming very slowly. In the breeding lagoons, about 50% of the dives are less than 1 min and 99% are less than 6 min, whereas dives longer than 12 min are associated with resting animals. Mothers, for example, typically float at or slightly below the surface for periods of up to an hour and then submerge for 5 to 10 min, or up to 26 min. When evading detection, grays often surface cautiously, exposing only the blowholes, exhale quietly without a visible blow, and sink silently beneath the surface (called snorkeling).
The species is active at the surface; spyhopping (raising the head vertically out of the water), breaching (leaping vigorously into the air), and other aerial behaviors (head stands with tails in the air, flipper slaps on the surface, etc.) are commonly performed by adults and older calves, especially on the breeding grounds (Fig. 7, also Fig. 4). Throughout dieir range, grays often appear to “play” and surf in or near the breakers and shallow water along shore. Some grays regularly rub themselves on beaches and sandbars on the breeding grounds and on die nibbing beaches off Vancouver Island. They also rub on pebble beaches and rocks in die Arctic, leaving behind shed barnacles. Some enter brackish water in fjords, coastal lagoons, and die moudis of rivers and emerge cleaned of barnacles and lice. Gray whales are noted to frequent places so shallow that diey appear to be lying on die bottom. Occasionally during the ebb tide, some are stranded (ap-parendy unharmed) until the incoming tide refloats them.
Figure 7 Cray whales breach frequently while migrating and on their winter breeding grounds. One animal was observed to breach 4C consecutive times.
G. Friendly Whale Behavior
Gray whales exhibit a sense of curiosity that appears early in life as calves investigate and “play” with floating objects such as balls of kelp and small logs. The whales, including mothers and calves, frequently approach whale-watching skiffs, particularly on the breeding grounds. Behaviors include stationing alongside the skiffs, rubbing against them, bumping, lifting, and blowing bubbles beneath the boats, and allowing the passengers to pet and stroke them (Fig. 8). This activity is popularly termed “friendly” behavior. In the lagoons, these curious grays seem to be initially attracted to the sounds made by the motors of the skiffs, which fall within the same frequency range as gray whale vocalizations. Since the first encounter with a friendly whale at the calving lagoons in the 1970s, friendly whales have become commonplace there and are also encountered to a lesser degree along the migratory route and even in the Bering Sea.
H. Predators and Mortality
Killer whales are the only predator of gray whales (besides humans), although several species of sharks, including the great white shark (Carcharodon carcharias) and tiger shark (Galaeo-cerdo cuvier), scavenge on carcasses and might kill a small number of calves. Pods of killer whales cooperatively pursue grays, especially calves and juveniles, and seem to attack by repeatedly ramming along their sides, grasping the flukes and flippers to immobilize and drown them, and trying to open their mouths to bite into the tongue. Killer whales have frequently been reported feeding on the tongues of gray whales and then leaving the carcasses as carrion. Sometimes grays turn on their back and slash out with a powerful tail to ward off the swift wolf-like packs of killer whales. Oddly, if cornered, they may go into “shock,” floating motionless at the surface, stomach up, while killer whales bite at the tongue and flippers. Rake-mark scars from teeth are often seen on living whales, indicating that many successfully ward off an attack. Some attacks may also represent practice or play by killer whales. A reduced risk of predation from killer whales (more abundant at high latitudes in colder coastal seas) might be a primary benefit to females leaving polar waters to give birth in the subtropies. However. predation pressure does not appear to be a significant determinant in the gray whale’s social organization.
Figure 8 A “friendly” gray tyhale cow and calf allow whale watchers to pet them (note the tip of the mother’s lower jaw in the foreground).
Other known causes of gray whale mortality include ship collisions, entanglement in fishing gear (particularly gill nets) and man-made debris, and whaling (legal aboriginal takes and poaching). Also, calves are sometimes severely struck by whales involved in courting/mating groups, which could result in accidental fatalities. No infectious diseases have been reported. Internal parasites occur but are not known to cause death. In 1999, 2000, and 2001 an unexplained, severe deterioration was seen in the physical condition and health status of some individuals in both eastern and western populations (gray whales were unusually thin, or emaciated). In the eastern population, mortality was unusually high, and some whales appeared to have died from starvation.
IV. Distribution, Migration, and Status A. North Atlantic Population(s) (Extinct)
The gray whale once existed on both sides of the North Atlantic. Complete and partial skeletons of grays that are subfossils (not yet mineralized) have been found on the east coast of the United States (from New Jersey to Florida) and in the eastern Atlantic from the Baltic coast of Sweden, the Netherlands, Belgium, and the Channel coast of England, the most recent dated from about 1650 a.d. (see Fig. 1). In the western Atlantic, the gray whale is thought to have migrated all along the Atlantic seaboard from Florida to Canada. The youngest North American specimen is from colonial times about 1675 a.d., whereas the oldest are around 10,000 years old. The European gray whale may have disappeared around 500 a.d., but there is a credible record for Iceland in the early 17th century. Evidently, based on written accounts, the last few grav whales in the Atlantic were exterminated by the late 17th or early 18th century, apparently by early Basque, Icelandic, and Yankee whalers. The disappearance of grays from both sides of the Atlantic coincides with the development of whaling, supporting the idea that overhunting in Europe. Iceland, and North America was responsible for, or at least contributed to, its demise.
B. Western North Pacific Population (or Korean-Okhotsk)
1. Distribution and Migration Historic records suggest that the western North Pacific population of gray whales formerly occupied summer feeding grounds in the Okhotsk Sea as far north as Penzhinskaya Bay and south to Akademii and Sakhalin-skiy Gulfs on the west and the Kikhchik River on the east (see Fig. 1). In autumn, the whales migrated south along the coast of eastern Asia from the Tatarskiy Strait to South Korea (passing Ulsan from late November to late January) to winter breeding grounds suspected to be along the coast of Guangdong Province and around nearby Hainan Island in southern China. The southern-most record was from the east coast of Hainan Island. The long-held belief that the western grays spent the winter on the soudi coast of Korea was unfounded. It was proposed that an additional migration corridor led down the east coast of Japan to winter breeding grounds in the Seto Inland Sea (where calving occurred) in southern Japan, but this is largely unsubstantiated. In spring, it is assumed that the whales undertook a reverse migration, passing back through the Sea of Japan to reach their summer feeding habitat in the Okhotsk Sea.
Today, the number of gray whales inhabiting the above region is severely reduced. Currently their only known summer-fall feeding ground is off the northeastern coast of Sakhalin Island. Russia. The winter calving and mating grounds are unknown, but may be in coastal waters of the South China Sea.
2. Exploitation and Population Status The western North Pacific gray whale was considered to be extirpated, or nearly so, during the 20th century but is known to survive today as a tiny remnant population. It is one of the most endangered and little-known whale populations in the world. This group was hunted intensely during the past three centuries, but its decline can be largely attributed to modern commercial whaling off Russia, Korea, and Japan between 1890 and 1960. Preex-ploitation abundance is unknown. Whaling pressure from the Japanese hand-harpoon fishery was underway by the 16th century. Japanese whalers continued to take grays in the 17th. 18th, and 19th centuries. A branch of the population speculated to have bred in the Seto Inland Sea of Japan was gone by 1900. Beginning in the 1840s, American and European whalers took grays in the Okhotsk Sea and western North Pacific until the early 20th century. The last major whaling period occurred between 1910 and 1933, when about 1400 whales were harvested bv Japanese and Korean whalers. The fishery dwindled as the whales ran out. and many authorities thought the population was exterminated. However, catch records for 67 whales taken from the Korean coast from 1948 to 1966 indicated that some western grays remained. From 1967 to 1975, a few were continuously caught. Sightings along the coast of Korea. Japan, China, and Russia after that were rare.
During the 1990s, a small number of gray whales were found feeding during summer and fall in the Okhotsk Sea, mostly along northeastern Sakhalin Island (in Russian waters north of Japan), emphasizing its importance as a feeding ground. The population size of western gray whales was estimated to be about 100 individuals in 1999 and less than 100 in 2001. The World Conservation Union listed this population as critically endangered in 2000. Some believe it is likely that the population is below a critical size sufficient for recovery and may soon become extinct: others suggest that it may be increasing slowly. There are no data from the population’s southern range off China, North Korea, South Korea, or Japan, and research is needed. It is generally agreed that the western and eastern gray whales are discrete geographical populations. Recent genetic work has documented pronounced differences between them (implying negligible levels of gene flow) and indicates that the eastern and western gray whales can be genetically differentiated at the population level.
C. Eastern North Pacific Population (or Califomia-Chukchi)
1. Distribution and Migration From the end of May through September, most of the eastern North Pacific population is on its summer feeding grounds in the shallow.
Continental shelf waters of the Bering Sea and Chukchi Sea (between Alaska and Siberia), the Beaufort Sea (east to 130°W), and the east Siberian Sea (west to 178°30′E) (Fig. 1). The range reaches its northern limit at 69°N at the edge of the zone of close pack ice (to Wrangel Island in some years). Access to the vast feeding ground is controlled by the seasonal formation, disintegration, and drift of ice (for 5-6 months it is ice covered). Gray whales are widely dispersed throughout much of the region, but the major feeding areas where they occur in greatest abundance are the northcentral and northwestern Bering Sea, as well as the western and southwestern Chukchi Sea. Although many of the feeding areas have not been studied. those that have are underlain by dense, infaunal amphipod communities. A highly preferred habitat is the Chirikov Basin (between St. Lawrence Island and Bering Strait). It contains one of the largest and most productive amphipod beds in the world and extends over 40,000 km”. Apparently, whales do not forage in the coastal waters on the eastern side of the Bering and Chukchi Seas, which is consistent with the lack of benthic amphipod infauna in that portion of the continental shelf. As a rule, grays are distributed in shallow waters near shore and rarely go beyond 50 km offshore, although they also aggregate on shallow fiats a great distance from shore (up to 180 km). The habitat utilized averages 38 to 40 m in depth, and from 1% to 7% ice cover, but can be as great as 30%. The grays are constantly moving; their distribution varies yearly, and even monthly, as a result of constant ranging between feeding areas. Their foraging areas also support the largest number of bottom-feeding marine mammals in the world, including walruses (Odobenus rosmarus), bearded seals (Erignathus barbatus). and sea otters (Enhydra lutris).
The departure of grays from the northern feeding grounds in late summer and fall is cued primarily by shortening pho-toperiods and ultimately necessitated by advancing ice formation over feeding areas as the Arctic summer draws to a close. Some turn southward as early as mid-August and begin the long migration extending 7500-10,000 km to the breeding grounds, depending on where they are on the feeding range. Starting in September, grays leave the Beaufort and east Siberian Seas and converge into the Chukchi Sea. In October and November, whales move south out of the Chukchi Sea into the Bering Sea. Then, whales travel southeast and exit the Bering Sea via Uni-mak Pass, Alaska (in the Aleutian Islands), the easternmost prominent corridor between the Bering Sea and the North Pacific Ocean. Some pass through as early as October, others as late as January, but 90% leave from mid-November to late December. Females in late pregnancy go first, followed by other adults and immature females, and then immature males.
Once through Unimak Pass, the whales travel along the coast of North America down to central California. The migration is spread out all along the coast of Canada and the United States. The main body of the population arrives in central California by mid-January and takes about 6 weeks to pass. Beyond Point Conception, California, the majority take a more offshore route across the southern California Bight, through the Channel Islands, and reencounter the coast in northern Baja California. When the last of the southward migrants reach central California in February, they begin to overlap with the first of the northward migrants returning to the feeding grounds.
From January to early March (through May for some cows and calves), most of the population is in the winter assembly area, which extends from about central California (Point Conception) southward along the west coast of the Baja California Peninsula and continues around Cape San Lucas to the southeastern shore of the Gulf of California off Sonora and Sinaloa, Mexico. Historically, a few continued on to Guadalupe Island, whereas others reached the Revillagigedo Islands. Although a few calves are born off California, most are bom along the open coast and in the calving lagoons and bays of Baja California and mainland Mexico. The principal calving areas (with 85% of the calves) are Scam-mon’s Lagoon (Laguna Ojo de Liebre), Black Warrior Lagoon (Laguna Guerrero Negro). San Ignacio Lagoon (Laguna San Ignacio), and the Magdalena Bay complex (from Boca de las Animas to Baln’a Almejas), all on the outer coast of the Baja California Peninsula. A few calves are also born on the mainland coast of Mexico at Yavaros in Sonora, and Baliia Refornia in Sinaloa.
The breeding lagoons penetrate far into desert regions through narrow entrances marked by lines of whitewater over barrier sand bars. Except for mothers and calves, however, the vast majority of gray whales in Baja California are outside the lagoons and estuaries in Baln’a Sebastian Vizcaino and Baliia de Ballenas and along the coastline, milling, courting, and wandering along the coast. Courting whales in general do not remain in the lagoons for extended periods. Rather, thev are constantly passing and repassing into and out of them, and roving to other areas of the winter assembly grounds, leading to a high turnover of courting whales and subadults in the lagoons. The activity of the grays continues unabated day and night. Cows with newborns seek the quiet, inner reaches of the lagoons early in the season, away from harassment by courting whales concentrated in the areas around the lagoon entrances and outside along the outer coast, where much rolling, splashing, and sexual play can be seen. However, cows with calves also move into the ocean (often at night) and then return during darkness ill morning hours, and some visit other lagoons within a season. As the consorting adults start their north migration, the mothers and calves essentially abandon the inner lagoon nurseries and occupy the area near the lagoon entrances. Some cows return to the same lagoon in successive years to bear their young, whereas others rear calves in various lagoons in different years.
The spring migration north to the Arctic feeding grounds begins in mid-February. It retraces the route of the fall migration, but is not as concentrated or as fast. Newlv pregnant females migrate first, returning soonest to the Arctic to feed in preparation for the high energetic cost of gestation and lactation. They are followed by anestrous females, adult males, and then immatures. Last to migrate are the mothers and calves; they remain in the breeding area 1-1.5 months longer than most grays while the calves strengthen and grow. The first journey to the Arctic is a time of particular danger for the calves, which are occasional targets of killer whales. Cows and calves tend to travel extremely close to shore (90% are within 200 in) and are mostly alone or in pairs. Northbound whales funnel into the Bering Sea through Unimak Pass from March through June.
The north migration culminates in the dispersal of gray whales throughout their Arctic feeding grounds, which is extended in time and closely related to the ice condition (spring melt). The earliest arrivals generally reach St. Lawrence Island by May as ice recedes north or when leads or polynyas (a large area of water in pack ice that remains open throughout the year) are extensive. The main core of the population usually arrives in the Bering Strait by the end of May, where they are distributed along the cracks of ice throughout areas free of pack ice. One part of the population moves southward along the Asiatic coast and another passes through the Bering Strait into the Chukchi Sea where the whales split off in two directions: east toward the Alaska Peninsula and west toward the Chukotka Peninsula. Another smaller route possibly runs toward the Asian coast, along the Aleutian and Commander Islands. By June, grays are common in the northern Bering Sea in ice-free years, and through the Bering Strait into the southern Chukchi Sea during summer and autumn, as well as into the northeastern Chukchi and Beaufort Seas. By August and September, the ice has retreated north an average of 480 km into the Chukchi Sea. Their eastern distribution in the Beaufort Sea is limited by pack ice, as is their western distribution in the Chukchi and east Siberian Seas.
The vast majority of gray whales go to the northern feeding grounds; however, a small but perhaps increasing number do not migrate the entire distance and spend the summer feeding along the coast from Baja California to British Columbia. These whales (called seasonal residents) join the southbound migrants again in early winter. Areas where they have been observed out of season in Mexico include Baln’a San Quintin and Cabo San Lorenzo, on the Pacific coast of Baja California, and Baln’a de Los Angeles in the Gulf of California.
2. Exploitation and Population Status Native peoples of North America and Siberia have taken gray whales from the eastern North Pacific population for thousands of years, and a few groups continue to hunt them today. The impact of aboriginal whaling was relatively slight, however, compared to the wholesale slaughter of this population by the first American and European commercial whalers to hunt them in the Pacific. In 1846, they discovered the winter breeding grounds of the gray whale, and commercial harvests began soon thereafter in the lagoons of Baja California, then along the migration route, and spread to the feeding grounds in the Bering Sea. From 1846 to 1874, it is estimated that a minimum of 11,390 grays (not including calves) were taken. From its inception, the relentless 19th century whaling, mainly by American whalers, devastated the population. The hunt in the breeding range was largely concentrated on the cows and calves that were easily killed in the crowded lagoons and bays. Because most of the cows carried fetuses, or would have been impregnated, or had calves that were killed or died of starvation, the reproductive capacity of the population was reduced greatly. By 1900, the once abundant population was thought to be nearly extinct, and whaling all but stopped due to lack of quarry. The attention of the whalers turned to other species, allowing the gray (perhaps a few thousand remained) a brief respite before the advent of modern whaling.
With the introduction of floating factory ships on the west coast of North America in 1905, the hunting of gray whales resumed. A few were taken off Baja California and California in 1919, but mostly between 1925 and 1929. About 48 were taken annually in the Bering Sea from 1933 to 1946. All together, at least 1153 were taken from the remnant population, mainly by Norwegian, Russian, Japanese, and United States vessels. Only fear of extinction led to their official protection in 1946, except for an aboriginal harvest of about 160 whales each year that have been taken legally by Siberian Eskimos, and also a few by Alaskan natives. Since receiving protection, and the end of research harvests of about 316 grays in the 1960s, the population has increased steadily (by 2.5% per year). Based on the most recent survey (in 1997-1998), the eastern North Pacific population was estimated to be 26,600, possibly exceeding the 1846 preexploitation abundance, which most experts place at between 15,000 and 24,000. There have been indications, however, that the population is approaching, or possibly exceeding, its carrying capacity and may have become food limited (large decreases in amphipod biomass have been linked to increased predator pressure from gray whales and to detrimental effects of global warming in the Arctic). If this is correct, we can expect the gray whale population to level off or even decline.
V. Conservation and Management
A. Legal Protection
Gray whales received partial protection from commercial whaling in 1931 under the Convention for the Regulation of Whaling (which was largely ineffectual). The major whaling nations, Japan and the former Soviet Union, were not signatories to this agreement. They continued to take grays until 1946, when they joined 15 other countries and ratified the International Convention for the Regulation of Whaling, which established the International Whaling Commission (IWC). The IWC was intended to provide for the proper conservation of whale stocks and thus make possible the orderly development of the whaling industry. Although it failed in its primary mission, one of its first actions was to officially halt commercial whaling for gray whales in 1946, while allowing native subsistence harvests and scientific collections. Nevertheless, there were violations of the agreement by member nations of IWC, as well as pirate whaling (whaling that is practiced by fleets that acted beyond any national jurisdiction). In 2000, Russian scientists revealed that “literally at every sighting” this prohibited species was illegally killed by the former Soviet Union from 1961 to 1979, and whaling statistics were falsified.
Gray whales were listed as endangered under the U.S. Endangered Species Conservation Act in 1969. Further protection was given by the Marine Mammal Protection Act of 1972 and the U.S. Endangered Species Act of 1973. Under the protection afforded by these and other measures, the eastern population of gray whales recovered. In 1994, it was removed from the List of Endangered and Threatened Wildlife and Plants (under the U.S. Endangered Species Act) when the population numbered 21,000. The population was also downlisted in the World Conservation Union’s “1996 IUCN Red List of Threatened Animals,” from “endangered” to “lower risk: conservation dependent.” However, changes to the listing of the eastern North Pacific gray whale had no bearing on the status of the western North Pacific gray whale population, which is still critically endangered.
There is no allowable commercial take of any gray whales. The IWC quota for the years 1998-2002 of 140 eastern grays annually (with an overall total of 620 in five seasons) is in response to the catch requested by the Russian Federation for its native people. It also includes an annual quota of five whales requested by the United States to satisfy the Makah Indian tribe’s tradition of whaling in Washington state. No grays have been allocated to Alaskan native hunters since 1991. Further protection for eastern gray whales was given by Mexico in 1972 when two of the principal breeding lagoons, Black Warrior Lagoon and Scainmon’s Lagoon, were declared the world’s first whale sanctuaries. The same status was extended to San Ignacio Lagoon in 1979. All lie within the Vizcaino Desert Biosphere Reserve, Mexico’s largest refuge, and entrance into the lagoons is regulated. Currently, not only is it illegal to hunt gray whales, it is also illegal to harm, harass, or even cause behavioral changes without special permits.
B. Concerns
Recently there has been a major shift in the physical environment of the Arctic region with wide-ranging effects on the biota, which may have a deleterious impact on gray whales. Over the past 20 to 30 years, there has been a trend of decreasing sea ice concurrent with increased sea surface temperatures due to global warming. Primary productivity has decreased an estimated 30-40% since 1976. Major declines of marine mammal, fish, and bird populations have occurred in the Arctic’s Bering Sea. Although the effects of climate warming on grays are unknown, there are indications that the depression in primary production may lead to reductions in the benthic prey communities on which they feed. Increased predation from the growing population of whales themselves also appears to be stressing the amphipod populations. The eastern North Pacific gray whales may be expanding their summer range in search of additional feeding grounds. Moreover, it is hypothesized that the increase in gray whale mortality in 1999 and 2000 included some whales that were starving. A substantial reduction in food resources, through anthropogenic or natural causes, could have long-term effects on the future health, growth, and stability of the gray whale population.
The region of the Okhotsk Sea around Sliakalin Island holds large reserves of oil and gas and is currently being developed jointly by Russian, Japanese, and U.S. companies; oil drilling and production activities plus increased shipping and aircraft traffic may cause physical habitat damage or disturb or displace the highly endangered western Pacific population of gray whales on their only known feeding ground.
Gray whales are intimately related to the coastal habitats in which they have evolved, and it is the dynamic nature of coastal regions that has shaped their unique life history and behavior. It is also precisely their coastal habits that place them in direct conflict with humans. It is not enough to stop overharvesting the whales, we must also protect their critical habitat and allow them living space. They cannot avoid exposure to our intensive coastal development, pollution, vessel traffic, military activities, noise, and industrial activities associated with increased exploration and development of continental shelf, oil, and gas resources over virtually their entire range. Additional concerns include disturbance from ecotourism along migration routes and within the calving grounds, entanglement in fishing gear (particularly gill nets), ship strikes, pollution from salt extraction facilities in Mexico’s gray whale refuges, and commercial developments in the breeding area of Magdalena Bay, Mexico. In a world where the human population is expected to double in the next century, the pervasive effects of the population explosion will lead to additional regional and global environmental problems and further approbation of living space and resources that the gray whale requires to sustain itself.