I. Filter Feeding and the Marine Environment
Fundamental necessity for any organism is acquiring sufficient food for maintenance, growth, and reproduction. This search for food likely drove the return of mammals to the ocean where they were able to exploit highly productive coastal waters. With their return to the sea. marine mammals evolved a number of foraging techniques. Filter feeding, found in mysticete whales and three species of pinnipeds (crabeater seals (Carcinophaga lobodon), leopard seals (Hydrurga leptonyx), and Antarctic fur seals (Arctocephalus gazella), is the most unique of these adaptations for feeding and is not found in any terrestrial mammals.
Filter feeding allows these marine mammals to exploit extremely abundant but small schooling fish and crustaceans by taking many individual prey items in a single feeding event. This adaptation arose in response to the unique patterns of productivity and prey availability in marine ecosystems.
Low-standing biomass and high turnover of small-sized primary producers that respond rapidly to nutrient availability characterize marine food webs. Due to spatial differences in the physical dynamics of marine ecosystems, productivity tends to be more patchy and ephemeral than in terrestrial systems. Consequently, marine grazers (e.g.. schooling crustaceans and fish) often occur in extremely high densities near patches of high primary production. Most marine mammals are primary carnivores and feed on these dense, patchily distributed aggregations of schooling prey. The spatial and temporal patchiness of this prey means that marine mammals must often travel long distances to locate prey, and the larger body size of marine mammals likely plays an important role.
Initially, thermoregulatory requirements selected for larger body sizes as mammals returned to the ocean. However, once dependent on marine prey, a large body size also provided a buffer for the patchy and ephemeral distribution of marine prey. Thus, larger individuals could endure longer periods and travel longer distances between periodic feeding events on patchy prey. While adaptive for exploiting patchy prey resources, a consequence of larger body size is a higher average daily prey requirement. For marine mammals that feed on patchy and ephemeral resources, this requires individuals to take in large quantities of prey during the short periods of time it is available.
Filter feeding is a foraging strategy that allows individuals to capture and process large quantities of prey in single feeding events, thus allowing them to acquire energy at high rates when small prey are aggregated. Indeed, for mysticetes. a large body size is probably a prerequisite for attaining a sufficiently large surface area for filter feeding. Thus, the interaction of availability of prey resources, high concentrations of prey in schools, and selection for large body size likely led to the evolution of filter feeding. Ultimately, a large body size and filter feeding allowed some marine mammals to exploit the extremely high densities of schooling prey that develop at high latitudes during the spring and summer, while fasting during the winter when these resources disappear. A large body size provided an energy store for wintering and long-distance migration without feeding.
Due to this dependency on patchy but extremely productive food resources, it is not surprising that filter-feeding whales are believed to have first evolved and radiated in the Southern Hemisphere during the Oligocene at the initiation of the Antarctic circumpolar current (ACC). It is generally agreed that the initiation of the ACC led to cooling of the southern oceans, increased nutrient availability, and thus increased productivity. This increased productivity provided a rich resource of zooplankton that could be exploited effectively through filter feeding.
Present-day filter-feeding marine mammals concentrate their foraging in polar regions and highly productive coastal upwelling regions. The Southern Ocean is still the most important foraging area for filter-feeding marine mammals. Prior to their exploitation by humans, the highest densities of mysticetes occurred in highly productive southern waters. Crabeater seals, Antarctic fur seals, and leopard seals are found primarly in the southern oceans where seasonally dense aggregations of krill develop.
II. Diet, Filter-Feeding Structures, and Prey Capture
All filter-feeding species feed on prey that form dense aggregations (primarily pelagic schooling fish and crustaceans or densely aggregated benthic amphipods). Two feeding adaptations have evolved to allow the exploitation of these dense aggregations: baleen (mysticete whales) and modified dentition (seals).
A. Seals: Diet, Feeding Morphology, and Behavior
Unlike mysticetes, pinnipeds evolved in the Northern Hemisphere where krill was not likely an important component of their diet. As a result, adaptations for filter feeding are not nearly as extensive in pinnipeds as in mysticetes.
Only three pinniped species regularly filter feed: crabeater seals, leopard seals, and Antarctic fur seals. When filter feeding, all three species feed almost exclusively on Antarctic krill, Etiphatisia superba, in the Southern Ocean where it is abundant and forms extremely dense aggregations. Of the three pinniped species, crabeater seals are the most highly specialized, with krill comprising up to 94% of their diet, whereas krill comprises approximately 33% of the diet of leopard seals and Antarctic fur seals. The most remarkable adaptation for filter feeding in pinnipeds is found in the dentition of crabeater and leopard seals. In both species, elaborate cusps have developed on the postcanines in both the upper and the lower jaws (Fig. 1). Once the mouth closes around a small group of krill, water is filtered out through the cusps, trapping krill against the insides of the modified teeth. Little detailed information is available on the behavior used by filter-feeding pinnipeds to capture prey. However, data from Antarctic fur seals indicate that they track the diel migration of krill: shallow dives are performed during the night and deeper dives during the day.
B. Mysticetes
1. Diet and Feeding Morphology Most mysticetes feed primarily on planktonic or micronectonic crustaceans (cope-pods and krill) and pelagic schooling fish found in shallow waters. The gray whale diet consists primarily of benthic apeliscid amphipods, although they can forage on a wide variety of prey, including schooling mysids in some areas. Right (Eubalaena spp) and bowhead (Balaena mysticetus) whales primarily feed on copepod crustaceans of the genus Calanus. All of the rorquals feed on euphausiids (krill) to some extent, and blue whales (Balaenoptera museidus) feed almost exclusively on euphausiids. The other rorquals have a more varied diet that includes copepods (sei whales—B. borealis) and schooling fish (minke—B. acutorostrata and B. bonaerensis, Biyde’s—B. edeni, humpback—Megaptera novaeangliae, and fin whales— B. physalus).
All mysticetes lack teeth and instead have rows of baleen plates made of keratin that project ventrally from the outer edges of the palate. Similar to fingernails, the plates grow continuously from the base, but are worn by the movements of the tongue. As the edges of the plates wear, hair-like fibrous strands emerge as fringes. The outer fibers of these fringes are coarser, whereas the inner fibers form a tangled fringe that overlaps with fringes on adjacent baleen plates. Rows of baleen plates form an extended filtering surface along each side of the palate.
The coarseness of the hair-like fibrous fringes, the density of fibers (number of fibers/cm”), the number of baleen plates, and the length of baleen plates vary among species and are related to the prey species captured in the filtering mechanism. Because gray whales (Eschrichtius robustus) feed primarily on sediment-dwelling benthic amphipods, they have the coarsest filtering mechanism, made up of about 100, 5-25 cm-long individual plates with veiy coarse fibers. This coarse filtering structure allows them to separate amphipods from bottom sediments. In contrast, right whales that feed on small copepods have a fine filtering mechanism composed of more than 350 baleen plates that can exceed 3 m in length. The fibers of right whale baleen are very fine, forming a dense mat capable of capturing copepods that are less than 5 mm long. The strong, flexible, and light characteristics of baleen plates made them commercially important in the 19th century where they served some of the roles of todays plastics.
Mysticetes have evolved three types of filter feeding: sediment straining (gray whales), skimming (right and bowhead whales), and lunging or gulping (rorquals). The morphology of mysticetes reflects these different strategies. Gray whale heads are straight and relatively short and contain short, coarse baleen and their throat regions possess only a few grooves (three to five) in the gular region that allows limited distention for taking in bottom sediment, water, and amphipods. Right and bowhead whale heads have a strongly arched rostrum that allows them to have very long and fine-textured baleen within a relatively blunt mouth. They have no throat grooves for distension and instead feed by swimming slowly and skimming prey from the water. Rorqual heads are large and contain enormous mouths that extend posteriorly nearly half of the total body length. Their mouths contain relatively short baleen that ranges from fine (sei whales) to medium texture (blue, fin, humpback, and minke whales). The heads and bodies of rorquals are much more streamlined than other mysticetes, allowing them to swim rapidly into a prey school to gulp large quantities of water and schooling prey. One of the most remarkable adaptations for feeding is the presence, in rorquals, of 70-80 external throat grooves. During gulping, these grooves open like pleats to allow the mouth cavity to expand up to four times in circumference, taking in a volume of water equivalent to about 70% of the animals’ body weight.
Figure 1 Dentition patterns in pinnipeds. Note modified cusps in postcanine teeth in filter-feeding crabeater and leopard seals.
2. Feeding Behavior Observations of feeding gray whales in the Arctic and Bering Sea have shown that the whales roll to one side and suck benthic invertebrate prey and bottom sediments, with some distension of the mouth cavity through the expansion of the throat grooves. Water and mud are expelled through the side of the mouth. A similar behavior is used by gray whales that do not migrate as far north where they feed on a variety of benthic invertebrates and schooling myscids.
This benthic foraging behavior creates scrapes of about Vz m deep and 1 by 5 m in shape in the ocean floor, and several studies have shown that the disturbance is an important factor in the ecology of soft-bottom benthic communities of the Arctic and Bering Seas.
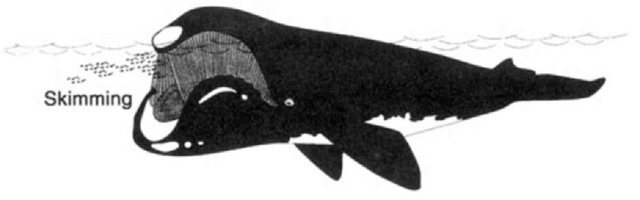
Right and bowhead whales forage by skimming with their mouths open through concentrations of crustaceans. As the whale swims, water and prey enter through the front of the mouth and water exits along the sides of the mouth while prey are trapped in the fine baleen (Fig. 2). Whereas right and bowhead whales generally feed singly, at times they may feed alongside one another—a V formation of 14 bowhead whales has been observed.
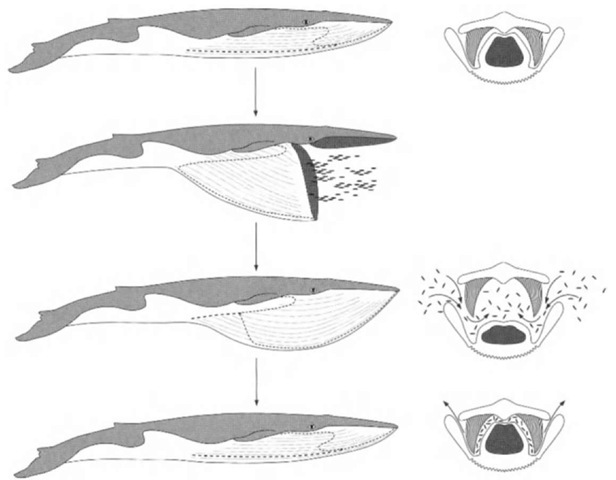
Rorqual lunge feeding has been described as the largest bio-mechanical event that has ever existed on earth. Rorquals capture food by swimming rapidly at a prey school and opening the mouth to gulp vast quantities of water and schooling prey (Fig. 3). To maximize the opening, the lower jaw opens to almost 90° of the body axis. This is possible because the lower jaw has a well-developed coronoid process. This process is located where the large temporalis muscle inserts and provides an anchor and mechanical advantage for control of the lower jaw while maximizing the gape for prey capture. It is not developed in other whale species, and a tendinous part of the temporalis muscle, the frontomandibular stay, enhances and strengthens the mechanical linkage between the skull and the lower jaw.
Figure 2 Skim feeding in right and boivhead ivhales.
With the mouth open, the onrush of water and prey is accommodated by the distending ventral pleats. The tongue in-vaginates to form a hollow sac-like structure (cavum ventrale) that lines the inside of the gular region and the ventral pleats distend fully. After engulfing entire schools of prey, the lower jaw is closed. The muscular tongue and the elastic properties of the ventral walls of the throat act in concert to force water out through the baleen (Fig. 3).
Although the process just described is fundamentally the same in all rorquals, some species exhibit modifications and additional adaptations. Sei whales skim feed in a manner similar to right whales, as well as feeding by lunging. Fin and blue whales often feed in pairs or trios that have a consistent echelon configuration. Humpback whales have a diverse diet and a wider variety of feeding behaviors. They have been observed bottom feeding and, while feeding on schooling fishes, have been observed to produce a cloud of bubbles and feed cooperatively to assist in prey capture.
Laboratory experiments have shown schooling fish to react to bubbles by aggregating more densely. Humpback whales appear to take advantage of this, as one member of a group of foraging whales produces a net of bubbles. The bubble cloud serves to aggregate and confuse the prey. Members of the group dive below the bubble cloud and surface together—one whale immediately adjacent to another. Group foraging humpbacks fonn long-term associations and the location of the whales in the surfacing group appears to be fairly constant through time. Humpbacks thus likely enhance prey capture success by using both bubbles and foraging cooperatively. A variation of bubble cloud feeding has been observed in humpback whales feeding on sand lance off New England. Here the bubble cloud feeding is followed by a tail slap—believed to cause the sand lance to aggregate more densely.
Figure 3 Lunge feeding in rorqual ivhales demonstrating expansion of the throat pleats in invagination of the tongue.
3. Feeding Ecology All filter-feeding whales exhibit distinct migration patterns linked to seasonal patterns in prey abundance. Seasonally dense aggregations of prey are probably necessary for successful filter feeding. For example, gray whales undergo the longest migration of any mammal: foraging during the summer and fall in the Bering Sea and Arctic Ocean when dense aggregations of benthic amphipods become available with the seasonal increase in productivity. Humpback whales seasonally migrate from breeding areas to higher latitude foraging areas where schooling fish and krill become seasonally abundant. The timing of coastal migration patterns of the California blue whale appears to be linked to annual patterns in coastal upwelling and krill development patterns.
Studies of the diving behavior and daily movement patterns of right whales have shown that they are found at dense aggregations of copepods, which in turn track oceanographic features such as fronts. Zooplankton densities in regions where right whales foraged in the southwestern Gulf of Maine were approximately three times the mean densities in the region (whale feeding densities averaged 3.1-5.9 g in-3, compared to 1.1-3.6 g m-3 where whales were not foraging). In a related study using hydroacoustic surveys, zooplankton densities where right whales were foraging were 18-25 g m~3 (compared to 1-5 g m-3 where whales were not foraging). Whale diving behavior is related to the depth of prey aggregations. In a year when copepods did not undergo diel migrations, dive depths averaged 12 in, with no dives exceeding 30 m throughout the day and night. In contrast, in a year when copepods showed strong diel shifts in depth (near the surface at night, deeper during the day), whale dive depths were significantly longer during the day.
Rorquals also track seasonal and diel patterns in the abundance and behavior of their prey. In general, the distribution and movement patterns of most rorquals consist of a seasonal migration from high latitudes where foraging takes place to low latitudes where they mate and give birth. However, data from blue whales in the Pacific indicate that feeding also takes place at low-latitude, “upwelling-modified” waters, and data from both the Pacific and the Indian Oceans indicate that some blue whales may remain at low latitudes year-round. Fin and blue whales foraging on krill off the coast of North America concentrate their foraging effort on dense aggregations of krill that are deep (150-300 m) in the water column during the day and may cease feeding when krill become more dispersed near the surface at night.
Rorqual foraging appears to only occur in regions of exceptionally high productivity, often associated with fronts, upwelling centers, and steep topography. It has been estimated that fin whales require prey concentrations of at least 17.5 g m-3 to meet daily energy requirements. Krill densities where humpback whales were foraging in Southeast Alaska have been estimated at 910 individuals m-3, and minimum required prey densities for humpbacks were about 50 individuals m~3. Krill densities in schools where blue whales were foraging in Monterey Bay, California, were estimated at 145.3 g m-3 compared to an overall mean density of zooplankton of 1.3 g m-3 in the area.
III. Summary
Filter feeding in marine mammals is an adaptation that allows individuals to take in large quantities of prey in one mouthful. This is particularly adaptive in marine ecosystems where prey are relatively small and often densely aggregated, but patchy and ephemeral in space and time. Most filter-feeding species feed on schooling fish and crustaceans. The large body size of marine mammals, particularly mysticetes, facilitates filter feeding by providing the ability to have a large filtering area relative to body volume. In addition, a large body size likely provides an energetic buffer for animals that must move long distances between dense prey patches and endure long periods of fasting between foraging events.