I. Marine Mammals in Captivity
Animals have been held in captivity in one form or another for hundreds, if not thousands, of years. Private collections turned into “public” collections. A private animal collection at Schloss Schonbrunn, Vienna, Austria, was opened to the public in 1765 and is considered to be one of the first modern zoos.
The first marine mammals to be held in captivity may have been polar bears (Ursus maritimus) and various pinnipeds. Reeves and Mead (1999) provide an excellent overview of marine mammals in captivity. Harbor porpoises (Phocoena phocoena) may have been held as early as the 1400s, polar bears since about 1060, and walruses (Odobenus rosmarus) since 1608. As with terrestrial animals, marine mammals were held in private collections.
However, most pinnipeds and some sirenians were not held in captivity until the late 1800s and early 1900s. Being considerably more difficult to capture, transport, and maintain, cetaceans, with few exceptions, have only been held in captivity since the mid-1900s. According to Reeves and Mead (1999), 4 species of sirenians, 33 pinnipeds, 51 cetaceans, the polar bear, and the sea otter (Enhydra lutris) have been held in captivity. Of these, 2 species of sirenians, 15 cetaceans, 22 pinnipeds, the polar bear, and the sea otter have reproduced in captivity. Among these, however, only a few species, such as the polar bear, California sea lion (Zalophus californianus), harbor seal (Phoca vi-tulina), bottlenose dolphin (Tursiops truncatus), and killer whale (Orcinus orca), have enough numbers and have been repro-ductively managed with enough production to be considered part of a successful captive breeding program.
Successful captive breeding of any marine mammal requires more than just holding the animals in a pool. Not only does it, as a bare minimum, require sexually mature males and females, but also adequate housing, nutrition, and consideration of the animals’ social needs. It becomes obvious when analyzing the history of successful births and survivorship of these species in captivity that early animal managers had little thought or, in some cases, knowledge of the requirements necessary for the development of successful breeding programs. Contrast past records of breeding and survivorship with recent trends beginning in the mid-1980s where captive breeding successes have equaled or, in some cases, surpassed the best scientific estimates of wild population breeding and survivorship and one can see just how far the captive marine mammal community has evolved. Detailed censuses of captive marine mammals in North America (Andrews et al, 1997; Asper et al., 1990) have shown the increasing numbers of captive-bred marine mammals, particularly California sea lions, harbor seals, and bottlenose dolphins. In 1995, 70% of the California sea lions, 56% of the harbor seals, and 43% of the bottlenose dolphins on display in North American facilities were captive-bom. This compares with 3, 4, and 6%, respectively, in 1975.
II. Why Breed Marine Mammals in Captivity?
A. Legal Necessity
In the “early days,” when animals were just being displayed as “curiosities,” it was easier to collect replacements when animals died. In most countries today this is simply not possible. Despite only a few species of marine mammals being threatened or endangered, they and their habitats are protected by a myriad of national and international laws and regulations. While a number of countries are considering regulation of the minimum conditions (e.g., pool size and volume, water quality, food quality and handling, medical care) under which marine mammals may be held in zoos, marine parks, or research facilities, apparently only the United States has such regulations in place. Even though the natural habitat of most marine mammals cannot be duplicated in captivity, the trend is toward larger, more complex, habitat-oriented displays and exhibits. Together, the various laws and regulations have reduced the collection of wild marine mammals and have eliminated smaller facilities that did not have the financial resources to adequately provide for their animals as required by law.
For obvious reasons, the just-mentioned laws and regulations favor captive breeding programs. A successful captive breeding program eliminates costly field expeditions and animal transports. Wild-caught animals are often of uncertain health status and may require routine treatments such as de-worming.
B. Maintaining/Enlarging a Captive Population
A successful captive breeding program can provide animals for other institutions with adequate holding facilities but without the financial resources to maintain a breeding colony or (if even possible) to collect wild animals. Captive-born animals have a known medical history and, to some extent, are imprinted on their keepers. Captive breeding programs have, out of necessity, reduced the impact 011 wild populations of marine mammals. Professional organizations such as the American Zoo and Aquarium Association (AZA) have established studbooks and other programs to assist with the management of captive animal breeding colonies. For example, studbooks track individual animals from birth to death and their reproductive histories. Computer programs are used to pair animals to optimize genetic diversity. In the United States, formal studbooks exist for the beluga whale (Delphinapterus leucas), common bottlenose dolphin. West Indian manatee (Trichechus manatus latirostris), gray seal (Halichoerus grypus), harbor seal, northern fur seal (Callorhinus ursinus), and polar bear and several others are under development. Similar studbooks are in place on other continents. The AZA also hosts a Marine Mammal Taxon Advisory Group whose function is to promote managed captive breeding of marine mammals.
C. A Breeding Program as a Scientific Resource
A successful captive breeding program is a unique scientific resource in that it allows one to document, in great detail, various aspects of reproductive behavior, reproductive physiology, and the subsequent birth, growth, and development of the offspring. This is particularly valuable for cetaceans, which are typically difficult to study in great detail in their natural habitat due to various environmental factors and the fact that they spend most of their lives underwater. It is, however, most important to recognize that studies on captive marine mammals, even in the best breeding colonies, do not and cannot replace field studies. Both types of studies (i.e., laboratory and field) are necessary to fully describe the biology of a species.
Routine components of a proper animal husbandry program include regular physical exams, collection of blood, urine, and fecal samples, body measurements, and body weights. These samples and data are virtually impossible to collect from wild marine mammals. Consider, for example, what it would take to get a daily urine sample from a wild bottlenose dolphin or killer whale! Captive animals are easily conditioned to provide urine samples and to station for blood sampling, body measurements, and so on. Figures 1, 2, and 3 illustrate the kinds of observations that can be made and the kinds of data that can be gathered.
D. A Breeding Program as a Conservation Resource
A successful captive breeding program may provide the physical and human resources necessary to save some species of marine mammals in imminent danger of extinction (see Ralls and Meadows, 2000). These resources may allow us to maintain a viable gene pool until the habitat can be restored or other reasons for endangerment are eliminated. While such an undertaking is certainly honorable and the right thing to do, the magnitude of the job should not be underestimated and there are, at the present time, obvious limits based in good measure on the sheer size of the animals. For example, the population of right whales (Eubalaena glacialis) in the western North Atlantic Ocean is about 350 animals and may be decreasing due to human activities. These animals may reach lengths of 18 m and weights on the order of 20 tons. No facility in existence today (or likely to be in the foreseeable future) could hold a breeding group of right whales. However, threatened or endangered marine mammals such as the Hawaiian and Mediterranean monk seals (Monachus schauinslandi and M. monachus, respectively) and the river dolphins (families Platanistidae, Iniidae, Pontoporiidae, and Lipotidae) could be maintained in viable captive breeding colonies.
Figure 1 Birth of a killer whale at SeaWorld Florida.
III. Assisted Reproductive Technologies
The domestic animal industry (cattle, hogs, horses) long ago realized that it is much more efficient to move genetic material (e.g., semen) to different facilities than it is to move the whole animal. Methodologies for semen collection, preservation, and transportation, along with methods for artificial insemination (Al), induction of ovulation, and even embryo transplantation, were developed and are in widespread use worldwide today. Some of the techniques have been applied successfully to endangered animals. It seems a logical next step in the captive breeding of marine mammals to apply some of these techniques.
The development Al, the most common assisted reproductive technology (ART), for commercial use began in the 1950s. The successful application of Al and other ART to domestic species and humans was in part because of their accessibility for research into their reproductive mechanism or reproductive physiology. Of critical importance for the successful application of ART in any species is the determination of how reproductive hormones and behavior relate to ovulation (Sorensen, 1979).
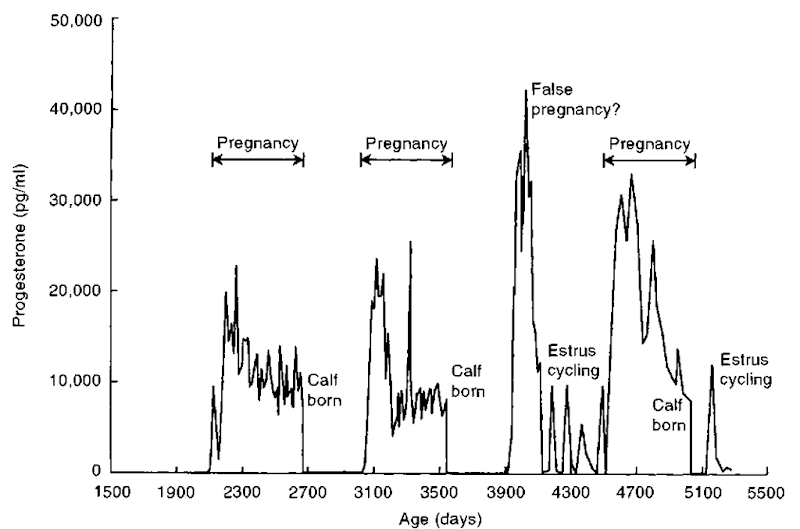
Figure 2 Semtn progesterone of killer whale from birth through sexual maturity and calving and showing estrous cycling and pregnancies. Progesterone levels were “zero” until after 1900 days of age.
Once ART were developed in domestic species, it was naively believed that they could easily be transferred to exotic species. However, relatively little success was realized with exotic animals until the late 1970s when an endocrinological breakthrough occurred. This breakthrough was the ability to analyze reproductive hormones in urine. This technology was successfully used to characterize the endocrinological events in a wide range of exotic species, including for the first time marine mammals, or the killer whale (Walker et al., 1988). The killer whale has proven to be easily trained to provide urine samples for endocrinologic evaluation and. as such, has provided detailed insight into its reproductive function. Despite this early success, no further attempts at applying ART to this species have occurred until recently.
The most common marine animal in captivity, the bottlenose dolphin, has been the subject of a few intensive investigations into its reproductive function. These investigations include the development of semen cryopreservation techniques and attempts at inducing or regulating ovulation (Robeck et al, 1994). Despite the information obtained, detailed information concerning endocrinological events as they relate to ovulation has not been obtained and, as a result, Al was never successfully developed.
What then are the needs for the development and application of ART to marine mammals? The most obvious ART that should be developed and the one that is most likely to have an immediate impact on the genetic management of captive marine mammals is artificial insemination. Once developed, Al would provide an immediate mechanism for marine mammal managers to increase the genetic fitness of their respective populations without having to rely on the transportation of animals between facilities. Wild animal population studies have shown that dolphins develop strong social ties to other animals and that these bonds can be maintained for the life of the animals. To what extent these bonds are important for the health of these animals can only be speculated, but it seems prudent for managers who have groups of compatible animals to hesitate changing the social structure by either removing or introducing more conspecifics. Further, bringing animals from other locations may expose the new population of animals to bacterial or viral organisms, which they have no natural resistance against.
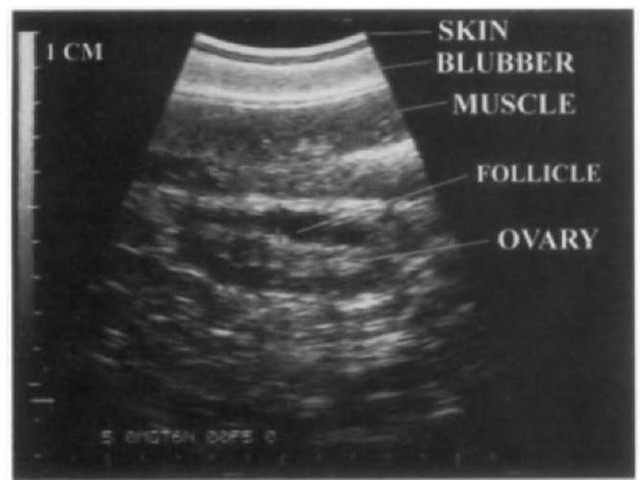
What steps are required to develop Al? As stated earlier, one must first be able to monitor, by visualization (surgically or through sonography) or endocrine methods, ovarian activity. Endocrine markers can predict ovulation only after they have been related to actual visualization of the ovarian activity. The ability to consistently find and observe ovarian activity transab-dominally with ultrasonography was first demonstrated in the bottlenose dolphin by Brook (1997). This simple technique has since been successfully utilized to consistently locate ovaries in many other captive marine mammals, including the killer whale, the Pacific white-sided dolphin (Lagenorhynchus obliquidens), and the false killer whale (Pseudorca crassidens) (Fig. 4). This ability then opens the door, with frequent body fluid sampling (most likely urine) for the identification of an effective endocrine marker of ovulation. Once ovulation can be predicted, then the ability to collect semen is an obvious requirement. Currently, only a few marine mammal species have been successfully collected on a regular basis. Early on during the process of trying to collect semen from these animals, electroejaculation was utilized with limited success. However, stress caused to the animals during collection and inconsistent results made this method unsuitable. Also, it was determined that bottlenose dolphins, animals that seem to have consistently elevated libidos, were relatively easily trained to provide semen via manual stimulation. Since the success with bottlenose dolphins, a killer whale and two Pacific white-sided dolphins have been trained to provide semen on a regular basis. Obviously, for Al to be widespread, all genetically valuable males should be trained to provide semen.
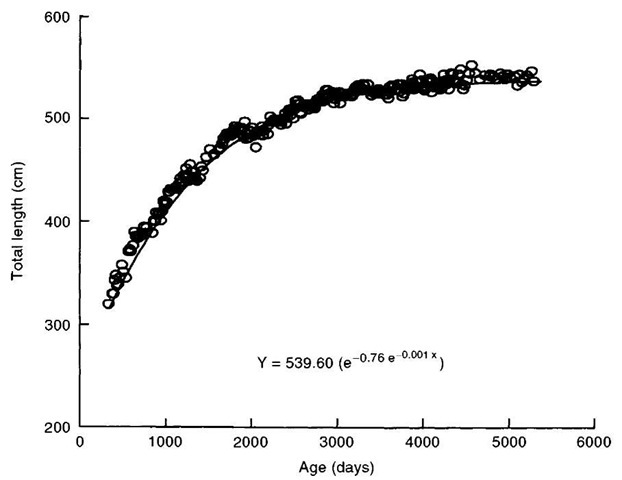
Figure 3 Killer whale growth (length) curve as an example of data that can be gathered from captive-bred animals.
Once the semen has been collected, it must be stored temporarily for immediate use or permanently by cryopreservation.
Figure 4 Sonographic image of an ovary of a Pacific white-sided dolphin showing a follicle.
Semen cryopreservation has been successfully accomplished by different methods (straws, pellets, or cryovials) in all of the species for which semen has been obtained. Although cryopre-served semen provides a long-term supply of genetic material from a particular animal and is the only method currently available to store semen for long (greater than a few days) periods of time, when used with Al it results in reduced conception rates. The reduced conception is believed to be the result of damage that occurs to the semen during the freezing process. As a general rule, cryopreserved semen must be deposited in the utenis and at close proximity to ovulation. Fresh cooled semen, having better motility and viability, can be placed with less accuracy, generally in the cervix, and with a greater time interval prior to ovulation. Thus, when attempting to develop Al, first attempts are generally made using fresh cooled semen.
As must be obvious from this discussion, a thorough understanding of female reproductive tract anatomy is essential to developing effective catheterization techniques for semen deposition. Despite the close phylogenetic relationship between captive delphinids, significant variation exists in their reproductive tract morphology. For example, bottlenose dolphins, being the best documented of captive species, have a pseudocervical vaginal fold or flap that must be traversed before encountering the true cervix. The space formed by the fornix surrounding the true cervix and the distance from the pseudocervical flap to the distal opening or external os of the cervix has been identified as the spermathecal recess. Deposition of fresh semen into this space (as opposed to vaginal placement) appears mandatory for a successful procedure. However, successful use of frozen semen for Al will probably require navigation through the pseudo and true cervix for uterine deposition. Knowledge of the anatomy of the bottlenose dolphin would do little to help placement of semen into the closely related member of the delphinid family, the Pacific white-sided dolphin. This species has a series of three annular folds that present anatomical barriers to the placement of semen. Finally, another member of the delphinid family, the killer whale, has a completely different arrangement consisting of two longitudinal ridged cervices in series.
Once the semen has been deposited and ovulation has occurred, how do you determine if you have had success? With the application of ultrasound for pregnancy detection, more and more marine mammal practitioners have become aware of the fact that all species of captive delphinids can exhibit variable periods of false pregnancies. These periods of elevated progesterone are currently endocrinologically indistinguishable from pregnancy. Thus, pregnancy can only be confirmed with the use of ultrasound.
Once Al has been developed successfully, other ART may begin to have effects in captive and potentially wild animal populations. One of the most intriguing developments in ART is the continued improvement of semen sexing techniques. Currently, semen sexing has been used to influence the sex ratio by close to 90% in either direction. However, the semen is damaged in the process, resulting in a significant reduction in viability. This translates into a requirement for interuterine or oviduct semen deposition. Unless or until the technique can be improved, semen deposition might be possible with laparoscopic techniques currently and tenuously being applied to cetaceans.
IV. Challenges for the Future
If self-sustaining populations are to be developed, then all reproductively capable animals must contribute to the gene pool. Producing viable offspring is the primary goal of any captive breeding program, but it must be managed to avoid overpopulation of the facility and to prevent or minimize inbreeding. This may require separate facilities for adult males, preparturient females, and females with new offspring. Breeding may be regulated simply by separating the sexes or by neutering (physically or chemically) both males and females.
Currently, many populations of marine mammal are kept in small groups or same-sex groups. Therefore, a large portion of reproductively mature animals within these groups are not reproducing. These animals are functionally excluded from contributing to the collective captive gene pool. Therefore, valuable financial resources and pool space are being used for a minority of the available genetic lines. This inefficient use of animal resources must be corrected before long-term population stability can be achieved. Judicious use of contraception and development of Al would help managers reach maximum utilization levels. However, if the maximum utilization of genetic resources does not result in a predictable stable population, then genetic infusions from wild stocks will be necessary. ART may provide another answer to this future dilemma if semen and possible oocytes can be collected from wild animals that are incidentally or purposely killed in fisheries or other commercial activities. These gametes can be placed into suitable captive animal recipients for reproductive purposes. As an example, researchers in Japan have created embryos by IVF (in vitro fertilization) from gametes collected postmortem from fin whales. Unfortunately, suitable captive recipients for this species do not exist. As an alternative to the ART solution or until ART were perfected, mangers could “borrow” adult males from wild populations for 1-2 years for breeding purposes and then return them. This, of course, involves considerable expense and there is no guarantee that any given animal would breed successfully.