Adaptation to the aquatic environment is a multiconver-„ ® gent phenomenon seen in a number of mammals. Most 1 fascinating is the degree of specialization in different groups that comprise the semiaquatic insectivores, otters, and pinnipeds, and the fully aquatic sirenians and cetaceans. Both the body shape and the morphology of the sensory organs and brain intimate which selective pressures may have led to exclusively aquatic life. There are some obstacles, however, in understanding brain evolution in aquatic mammals. First, we are only marginally familiar with brain morphology of a very few species, and here mainly the bottlenose dolphin (Tursiops truncatus; Figs. 2-3). Second, the brain itself does not fossilize; only the outer shape can be studied in natural endocasts, and these are biased by covering blood vessels, meninges, and geological artifacts. Thus, the tracing of brain evolution in fossils is difficult and should be supplemented by phylogenetic reconstruction on die basis of extant relatives. This is particularly tme for the cetaceans and the lack of adequate data from their closest relatives, the ungulates (hoofed animals; Fig. 1) and the more distantly related paenungulates [hyraxes or conies, sirenians or sea cows, and pro boscideans or elephants; for a survey see Berta and Sumich (1999)]. Third, although the comparative consideration of analogous developmental trends (primates) may be useful for the understanding of brain evolution in highly encephalized aquatic mammals, the paucity of data often leads to an overestimation of these analogies. Particularly the large size of the cetacean brain sometimes has led to an anthropocentric approach to these mammals, thereby resulting in inadequate questions and wrong answers. This article first focuses on the cetacean, particularly the odontocete brain, and then discusses its specifics together with those of convergent adaptive trends seen in semiaquatic and other aquatic mammals so as to provide an idea of what may have happened dining the evolution of these mammals.
Among the most fascinating characteristics of cetaceans are their exceptionally large brain, both in absolute and relative terms, and their extremely convoluted neocortex. Whereas dolphins usually have a brain mass of about 200-2000 g, the maximal size is attained in killer whales (Orcinus orca) and sperm whales (Physeter macrocephalus) with nearly 10,000 g. Basically, cetacean brains show the typical mammalian bauplan and are as complicated morphologically as those of other mammalian groups. To some extent they parallel the simian and human brains. In this respect, however, it has to be kept in mind that cetaceans have been subject to profound modifications in brain morphology and physiology during 50 million years of separate evolution in the aquatic environment. Moreover, it is still very difficult to correlate the results of behavioral and physiological research on dolphins with the existing neuroanatomical data. Because invasive experimentation is not possible in cetaceans, the functional significance of such data can only be elucidated via comparison with other aquatic or terrestrial mammals.
Most studies during the last decades have focused on the morphology and physiology of the adult odontocete brain and its functional systems [for reviews, see Jansen and Jansen (1969), Pilleri and Gihr (1970), Morgane and Jacobs (1980), Glezer et al. (1988), and Ridgway (1986, 1990)]. Concerning the development of the cetacean brain, very few recent papers have been dedicated to the striped dolphin (Stenella coeruleoalba; Kamiya and Pirlot 1974), harbor porpoise (Phocoena phocoena; Buhl and Oelschlager 1986), pantropical spotted dolphin (Stenella attenuata; Wanke 1990), narwhal (Mon-oclon monoceros; Holzmann (1991), and sperm whale (Oelschlager and Kemp 1998). Some studies of the morphology and ultrastructure of the cetacean cortex are by Supin et al. (1978) and Manger et al. (1998), Other relevant publications are those by Bauchot and Stephan (1966) on semiaquatic insectivores; Schwerdtfeger et al. (1984), Schulmeyer (1992), and Marino (1998) on the encephalization of toothed whales and the quantitative composition of their brain; and Pirlot and Kamiya (1985), Reep et al. (1989), and Reep and O’Shea (1990) on the brain of manatees.
I. Morphology of the Cetacean Brain A. General Appearance of the Brain
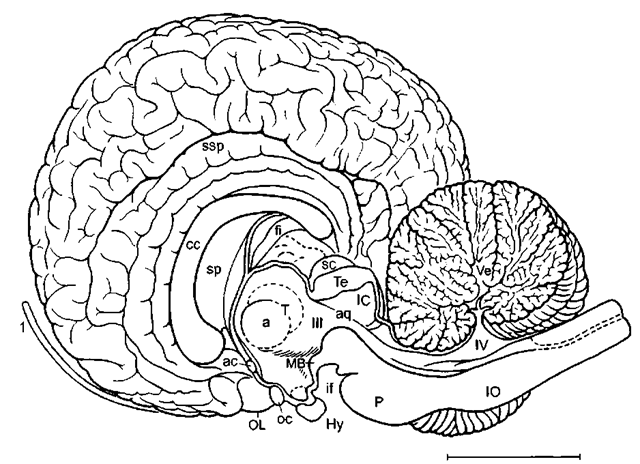
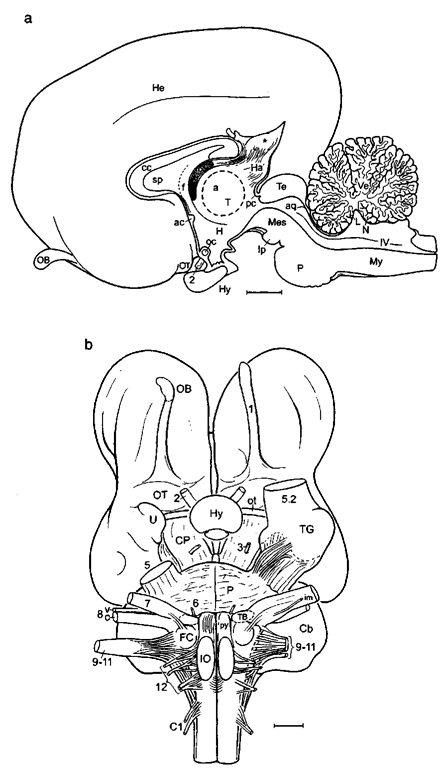
Whereas its development in the embryonal and early fetal period is similar to that of other mammals, the brain of adult whales and dolphins is rather spherical in comparison with that of generalized land mammals (Fig. 1) and is somehow reminiscent of a boxing glove (Fig. 2a). In correlation with the so-called “telescoping” of the skull along the beak-fluke axis, both the cranial vault and the brain are short but wide (Figs. 2-7), and more so in the toothed whales (odontocetes) than in baleen whales (mysticetes). In the bottlenose dolphin (Fig. 2), the hemispheres are rounded and high, and the anterior profile is rather steep. In ventral aspect, the contour of the odontocete forebrain is more trapezoidal, whereas in mysticetes it is more trilobar, with the area of the insula being visible as an indentation between orbital and temporal lobes (Figs. 3, 4, and 7). In comparison with hoofed animals (Fig. 1), the telencephalic hemisphere seems to be rotated rostralward and ventralward leading to a subvertical position of the corpus callosum (Figs. 2b and 6). In some odontocetes (bottlenose dolphin, sperm whale), the posterior myelencephalon and the anterior spinal cord arch around the cerebellum. Via an S-shaped transition, the spinal cord then continues straight along the body axis, thus accounting for the shortening of the cetacean neck region.
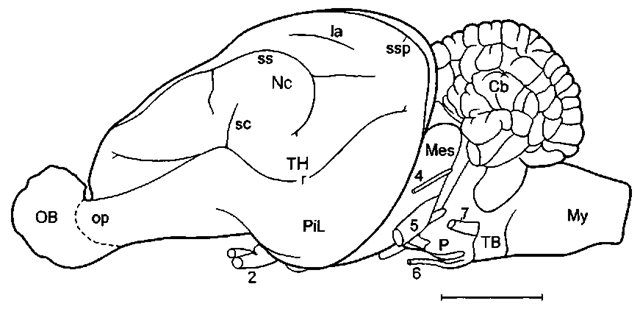
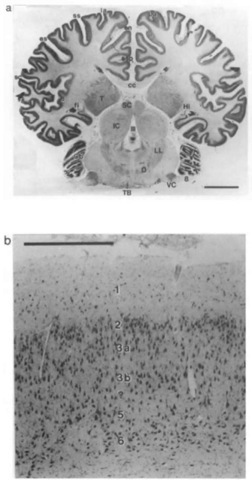
Figure 1 Lateral aspect of the brain of a generalized land mammal, the mouse deer (Ilyemoschus aquaticus), as a representative of the hoofed animals. Here the telencephalic hemisphere (TH) is rather flat, the neocortex (Nc) is moderately folded, and the olfactory bulb (OB) and the olfactory cortex in the piriform lobe (PiL) are large. In more advanced mammals such as cetaceans (see later), the hemispheres are much larger, the neocortex is much more extended, i.e., extensively folded, and it strongly dominates the olfactory/ cortex, which then is only found on the rostral basal surface of the telencephalic hemisphere. Cb, cerebellum; la, lateral sulcus; Mes, mesencephalon; My, myelencephalon; op, olfactory peduncle; P, pons; r, rhinal sulcus; sc, Sylvian cleft; ss, suprasylvian sidcus; ssp, sple-nial sulcus; TB, trapezoid body; 2, optic nerve; 4, trochlear nerve: 5, trigeminal nerve; 6, abducent nerve; 7, facial nerve. Scale: 1 cm. Modified after L. Sigmund (1981; Vest Cs. Spolec. Zool. 45, 144-156).
The ventricular system reflects the foreshortening of the brain in the tight coiling of the lateral ventricles, the shortness of the fronto-orbital region (anterior horn), the lack of an occipital pole of the hemisphere (no posterior horn), and the large size of the midbrain (cerebral aqueduct). Only rarely is a posterior horn of the lateral ventricle reported.
B. Telencephalon
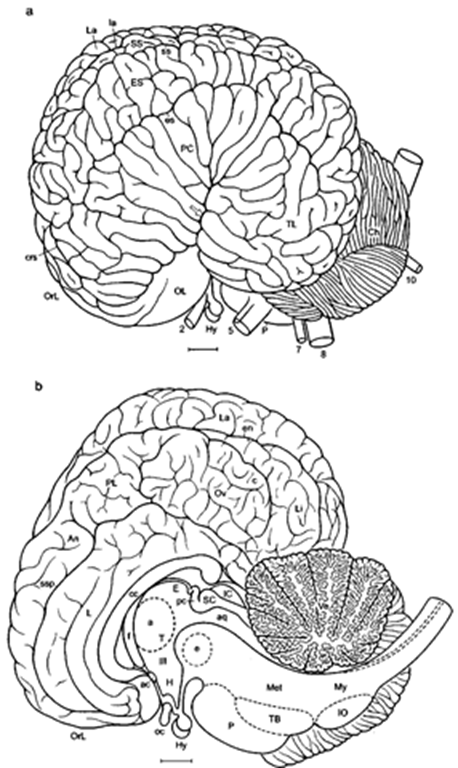
1. Cortex In comparison with generalized tetrapod mammals (Fig. 1), the surface of the telencephalic hemispheres is extremely convoluted, particularly in toothed whales (Figs. 2-7). Gyrification in baleen whales is less extreme because of the greater width of their cortical layers. The neocortex accounts for Figure 2 Bottlenose dolphin brain: (a) lateral [after Langworthy (1932), modified after Morgane and Jacobs (1972) and Pilleri and. Gihr (1970)] and (b) another specimen, mediosagittal aspect (after Morgane and coworkers). Cortex and structures containing nuclei are labeled with capital letters, fiber tracts (white matter) and sulci with small letters. Arrow pointing into sylvian cleft; a, interthalamic adhesion; ac, anterior commissure; An, anterior lobule; aq, cerebral aqueduct; c, “calcarine” cleft; cc, corpus callosum; Ch, cerebellar hemisphere; crs, cruciate sidcus; e, elliptic nucleus; E, epithalamus; en, entolateral sulcus; es, ectosylvian sulcus; ES, ectosylvian gyrus; f fornix; H, hypothalamus; Hy, hypophysis; IC, inferior colliculus; IO, inferior olive; L, limbic lobe; la, lateral sulcus; La, lateral gyrus; Li, lingual lobule; Met, metencephalon; My, myelencephalon; oc, optic chiasm; OL, olfactory lobe; OrL, orbital lobe; Ov, oval lobule; P, pons; pc, posterior commissure; PC, perisylvian cortex; PL, perilimbic lobe; SC, superior colliculus; ss, suprasylvian sulcus; SS, suprasylvian gyrus; ssp, suprasplenial (limbic) sulcus; T, thalamus; TB, trapezoid body; Ve, vermis; 2, optic nerve; 5, trigeminal nerve; 7, facial nerve; 8, vestibulocochlear nerve; 10, vagus nerve; III, third ventricle. Scale: 1 cm. the large size of the telencephalon and thus the large size of the brain [percentage of the neocortex: 63% in the franciscana (La Plata dolphin or Pontoporia blainvillei); 87% in the sperm whale.
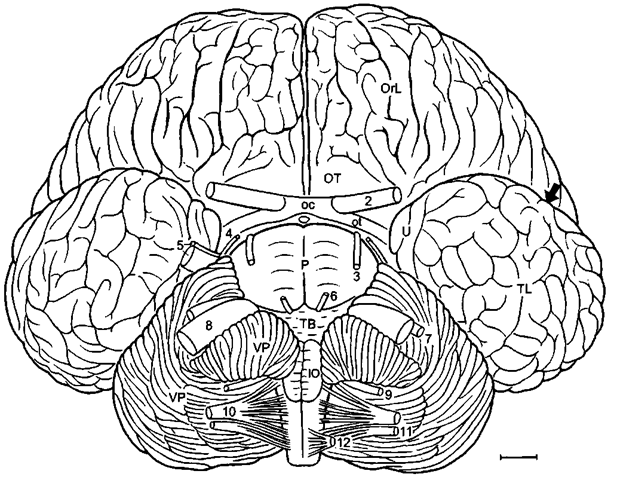
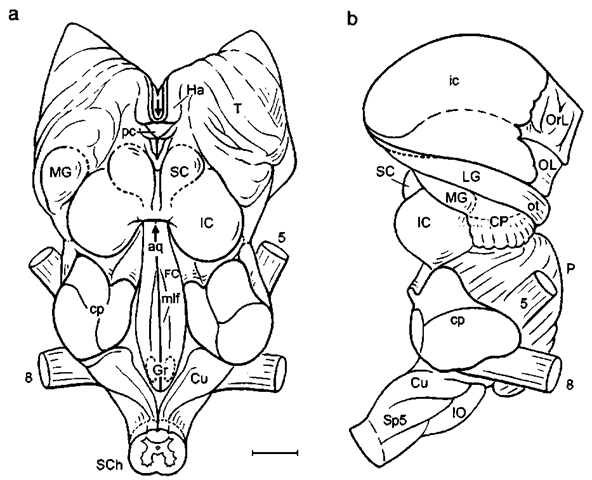
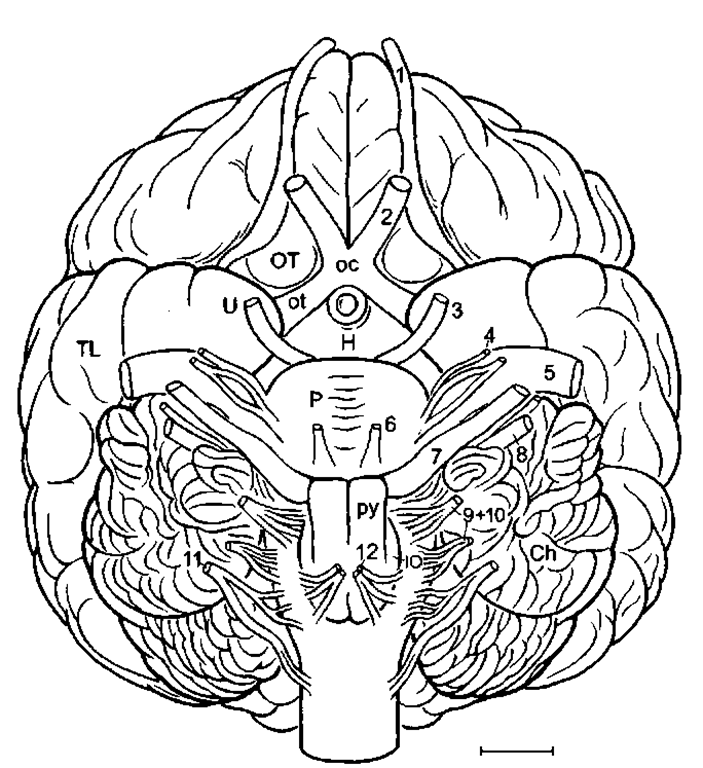
Figure 3 Bottlenose dolphin brain in basal aspect. Arrow pointing into sylvian cleft, ot, optic tract; OT, olfactory tubercle; TL, temporal lobe; U, uncus; VP, ventral paraflocculus; 2-12, cranial nerves; 3, oculomotor nerve; 4, trochlear nerve; 6, abducens nerve; 9, glossopharijngeus nerve; 11, accessory nerve; 12, hypoglossals nerve. Scale: 1 cm. After Langworthy (1932), modified after Pilleri and Gihr (1970) and Morgane and Jacobs (1972).
As in higher primates, the cortex of the cetacean olfactory and limbic systems (allocortex) is restricted to the rostral base of the hemisphere (paleocortex; olfactory system) or is located at the inferior horn of the lateral ventricle in the temporal lobe (archicortex: hippocampus as part of the limbic system). The archicortex in cetaceans, above all in toothed whales (Fig. 5X is much smaller than in terrestrial mammals. This correlates well with the small size of other components of the limbic system, e.g., the fornix as the main fiber tract of the hippocampus and the mammillary body as a main relay within the limbic system, whereas the cortical fields above the corpus callosum ("limbic lobe") and the entorhinal cortex on the temporal lobe are well developed. As in primates, the cortex of the cetacean limbic lobe presumably does not have an immediate relationship to olfaction. In adult baleen whales, the nose is small but obviously functional, and the same holds true for the components of the rhinencephalon. In adult toothed whales, these components are very much reduced: there is no olfactory part of the nose, olfactory bulb or tract, and the central parts of the olfactory system are small.
a. surface configurations. The fissural or gyral pattern of the cetacean cortex, which has been discussed in many papers in the past, bears general resemblance to that of carnivores and ungulates (Figs. 1-7 and 12). On the convex lateral surface and the vertex of the hemisphere, the main fissures (ec-tosylvian, suprasylvian, lateral sulcus) run at different distances around the Sylvian cleft. Thus, the ectosylvian gyrus is bordered by the ectosylvian and suprasylvian sulci, the suprasylvian gyrus by the suprasylvian and lateral sulci, and the lateral gyrus by the lateral and entolateral (paralimbic) sulci. As in other high-encephalized mammals, the insular area (Figs. 4 and 5) is covered by so-called "opercula" of the neighboring neocortex, which meet at the lateral hemispheral fossa (Sylvian cleft) and are com- bined in the term perisylvian cortex (Fig. 2a). The medial cortex of the hemisphere (Fig. 2b) is subdivided by the suprasplenial sulcus or limbic cleft. The cruciate sulcus (Figs. 2a and 12) separates an anterior (medial) motor cortical field from a posterior (lateral) somatosensory field and is therefore a candidate for ho-mologization with the ansate sulcus in hoofed animals as well as the central sulcus in primates. It is doubtful whether die "cal-carine sulcus," which originates from the entolateral paralimbic cleft and encircles the oval lobule (Fig. 2b), is the honiologue of the primate calcarine fissure that houses the primary visual field. Electrophysiological mapping experiments in dolphins have detected visually responding cortical fields in a more anterior and lateral position on the vertex of the hemisphere.
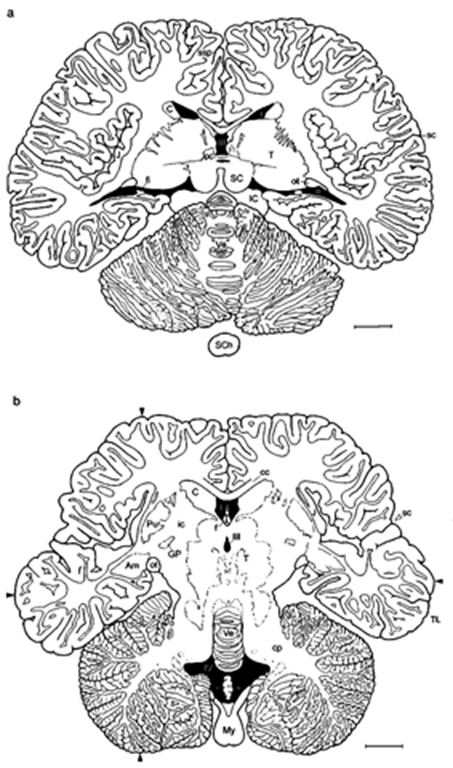
Figure 4 Common dolphin, Delphinus delphis (horizontal sections): (a) through middle of brain and (b) through basal brain. Arrowheads: locations for measu rement of length and width of the brain, ventricular spaces black. Am, amygdaloid body; C, caudate nucleus; cp, cerebellar peduncles; ft, fimbria; GP, globus pallidus; J, insula; ic, internal capsule; Pu, putamen; sc, sylvian cleft; SCh, spinal cord; ssp, suprasplenial (limbic) sulcus; I-IV, ventricles. Scale: 1 cm. Modified after Pilleri et al. (1980).
Figure 5 La Plata dolphin: transverse section of 20 thickness through adult brain. Cresyl violet stain, (a) Overview and (b) cortex sample from the lateral gyrus. Cb, cerebellum; Hi, hippocampus; LL, lateral lemniscus nuclei; O, superior olive; VC, ventral cochlear nucleus; 8, vestibulocochlear nerve. Numbers 1-6 in insert, layers I-VI of the neocortex. Scale: (a) 1 cm and (b) 500 |xm.
In one of the most plesiomorphic whales (Susu or Ganges river dolphin; Platanista gangetica), gyrification is still relatively simple. In the dorsal aspect, the main fissures are straight, smooth, and similar to the mesonychid Synoplotherium, a fossil terrestrial relative of the cetaceans (Geisler 1998). Brain length in the latter still exceeded brain width, the formation of a temporal lobe had only just begun, and the olfactory system was well developed. Archaic fossil cetacean (archaeocete) brains are difficult to interpret morphologically because they apparently had large retia mirabilia on the surface of the brain as is seen in living baleen whales, which largely conceal the posterior (cerebellar) part. Their telencephalic hemispheres were obviously small and showed no signs of gyrification. As a result, the pattern of gyrification in cetaceans is not necessarily homologous in detail to that of their potential terrestrial relatives, the extant hoofed animals (Fig. 1).
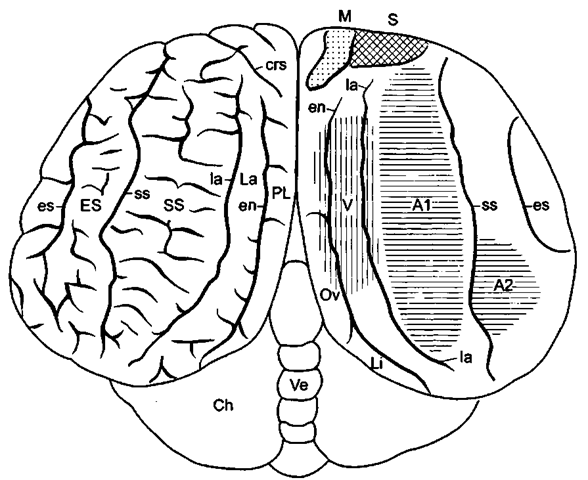
b. localization of cortical areas. Electrophysiological cortical mapping experiments in the bottlenose dolphin located the motor neocortical field in the frontal (orbital) lobe rostral to the paralimbic lobe (Fig. 12). The motor cortex is characterized by the presence of giant pyramidal neurons and gives rise to the pyramidal tract. The somatosensory field is situated rostral to the visual and auditory fields. The position of the visual fields is somewhat more complicated. Although different in many aspects from other mammalian visual cortices, those of the dolphin are apparently well developed and highly differentiated. Whereas all authors place the visual cortex in the lateral gyrus, some distinguish a primary visual field near the medial border of the suprasylvian gyrus from a secondary field in the lateral gyrus. Other authors find an additional visual area in the medially adjacent part of the paralimbic lobe (Fig. 12). On the basis of histological analysis, a visual field has also been reported for the area along the supposed "calcarine" sulcus that separates the oval and lingual sublobules of the paralimbic lobe (Fig. 2b). The large primary auditory field lies on the vertex of the hemisphere in the suprasylvian gyrus and lateral to the visual field(s), and the secondary auditory field lies more laterally in the medial part of the ectosylvian gyrus.
Viewed as a whole, the topography of the motor and sensory projection fields of dolphins differs from that in other mammals. However, the primary cortical fields in cetaceans have retained the sequence found in plesiomorphic terrestrial mammals. Therefore, it seems as if the hemisphere would have been expanded to such a degree in a caudal and ventral direction (huge temporal lobe) that the auditory cortex extends as a belt along the vertex of the hemisphere and reaches further caudally in the dolphin than the visual field, which now is located more in the center of the hemisphere. In contrast to carnivores (cat, dog, seal), however, where all of the auditory cortex is located in the ectosylvian gyrus, this holds only for the secondary auditory field in toothed whales. The lateral surface of the whale hemisphere may be interpreted as a large "association cortex" connecting the auditory fields with other sensory and motor modalities.
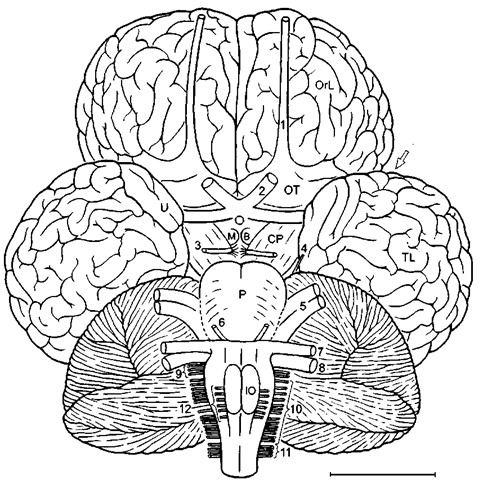
Figure 6 Brain of humpback whale (Megaptera novaeangliae) in mediosagittal section, if, interpeduncular fossa: sp, septum pellucidum; Te, tectum; 1, olfactory nerve. Scale: 5 cm. After Breathnach (1955, I960).
Figure 7 Brain of humpback whale in basal aspect. CP. cerebral peduncle; MB. mammillarij bodies. Scale: 5 cm. After Haug (1970), modified after Breathnach (1955), Pilleri (1966), and Pilleri and Gihr (1970).
2. Commissures The size of the commissural systems is specific for cetaceans and shows correlations with cortical and nuclear structures throughout die forebrain (Figs. 2b, 4-6, and 8a).
The anterior commissure, which links neocortical and pale-ocortical areas of both temporal lobes, obviously is weak due to the strong reduction of the olfactory system. The corpus callosum as the main link between the neocortical fields of both hemispheres is rather thin in dolphins relative to total brain mass and in comparison to the situation in other mammals. Tarpley and Ridgway (1994) have shown that in cetaceans the cross-sectional area of the corpus callosum (defined by its area in the midsagittal plane) related to brain mass generally decreases in larger-brained toothed whales (and pinnipeds), thereby suggesting that increases in brain weight are not necessarily accompanied by increases in callosal linkage between the telencephalic hemispheres. Thus, the corpora callosa of a killer whale and a human brain show the same cross-sectional area, with the killer whale brain being some five times heavier than that of the human. Furthermore, this relative reduction of the corpus callosum in larger species obviously has not been compensated by an enlargement of other commissural tracts. In conclusion, the interhemispheric connectivity seems to correlate inversely with brain weight insofar as larger brains possess a lower neocortical neuronal density with the result that relatively fewer fibers constitute the corpus callosum. An additional explanation for the regression of the corpus callosum in larger brains could be a smaller percentage of cortical neurons establishing interhemispheric connections and thus a certain independence on the part of both hemispheres. In elec-troencephalographic experiments, sleeping bottlenose dolphins have been reported to show signs of wakefulness (low voltage, fast activity waveforms) in one hemisphere and sleep (high voltage, slow wave) in the opposite hemisphere. The posterior commissure (Fig. 8a) is very well developed. Provided that its connections to other brain structures are similar to those in other mammals, the considerable size of the posterior commissure in cetaceans may suggest massive projections from the somatosensory relay nuclei of the brain stem and the cerebellum to the contralateral pretectum and thalamus.
3. Basal Ganglia All components of the basal ganglia as known from other mammals are present in cetaceans (corpus striatum, globus pallidus, claustrum, amygdaloid complex) and, for the most part, show the usual topographic relationships to each other and to neighboring structures (Fig. 4). Moreover, their histological organization corresponds well with that in other mammals. The caudate nucleus as the main part of die large corpus striatum, which bulges distinctly in the area of the "olfactory tubercle" (olfactory lobe), together with the putamen, is largely separated from the latter by a well-developed internal capsule.
Figure 8 Brain stem of the bottlenose dolphin: (a) dorsal and (b) lateral aspects, cp, cerebellar peduncle, CP, cerebral peduncle; Cu, cuneate nucleus; FC, facial collicidus; Gr, gracile nuclei; Ha, habenula; LG, lateral genicidate body; MG, medial geniculate body; mlf medial longitudinal fascicle; pc, posterior commissure; Sp5, spinal nucleus of trigeminal nerve. Arrows pointing into cerebral aqueduct (aq). Scale: 1 cm. After Lang-worthy (1931), modified after Pilleri and Gihr (1970), and Morgane and Jacobs (1972).
Reports regarding the size of the basal ganglia in cetaceans are contradictory. Schwerdtfeger et al, (1984) have shown that in the franciscana (La Plata dolphin), the corpus striatum, one of the most important centers for locomotion, is large and attains a size index between that of prosimians and anthropoids. Moreover, as in primates, a size correlation between the striatum and the neocortex seems to be valid for the dolphins as well. The stnicture of the amygdaloid complex resembles that of other mammals very closely. The size of the amygdala as a whole seems to have been affected only slightly by the reduction of the paleocortex in odontocetes, giving the impression that this nucleus, as in primates, is largely independent of the olfactory system. Its relative size in the franciscana seems to be larger than that in primates, presumably on account of its interconnections with the hypertrophied auditory system and the temporal lobe. However, the cortieomedial group of the amygdaloid nuclei, which functionally depends largely on the olfactory system, occupies the same proportion of the entire amygdaloid complex in the harbor porpoise as in the macrosmatic sheep. The lateral amygdaloid nucleus may bear some relation to auditory function, as this nucleus is extremely well developed both in whales and in bats.
C. Diencephalon
The relative size of the diencephalon in plesiomorphic dolphins (franciscana; Ganges river dolphin) is approximately the same as that in simian monkeys, including the human. There are no reliable data for advanced, i.e., pelagial, dolphins. The predominant structure is the thalamus (Figs. 2b, 4a, 5, and 6). The shape of the diencephalon in mediosagittal aspect is often rather wedge-like in adult cetaceans, with the hypothalamus bending slightly caudalward and tapering in the direction of the hypophysis, particularly in larger toothed whales. Whereas in late embryos and early fetuses the floor of the hypothalamus is rather long, it is foreshortened rapidly in later stages during the telescoping process, especially in larger toothed whales. Thus, the transverse interpeduncular fossa between the optic chiasm and pons appears slit-like in adult toothed whales, whereas it is much wider in baleen whales (Fig. 2b, 3, 6, and 7).
1. Epithalamus The habenular complex is large and the habenular commissure well developed. The pineal organ is reduced or even lacking in cetaceans. A pineal rudiment is present between the habenular and posterior commissures in embryos and early fetuses of dolphins and the sperm whale, but not in the early fetal nanvhal (Holzmann, 1991). In adult whales and dolphins, many observers found the pineal organ to be lacking; rudiments, however, have been found in the humpback whale (Megaptera novaeangliae) and fin whale (Balaenoptera physalus).
2. Thalamus Basically, the organization of the large thalamus in cetaceans corresponds well with that in a variety of terrestrial mammals, among them ungulates and primates. There are four groups of nuclei in the dorsal thalamus that constitute about 92% of the thalamus in the bottlenose dolphin: anterior, medial, ventral, and lateral. (1) The anterior group of nuclei, which is related to the cortex of the highly developed cetacean limbic lobe, is well developed, but constitutes only a small part of the total dorsal thalamus. The anteroventral nucleus, which projects to the anterior limbic cortex, dominates this group. In contrast, the mammillary body and the interconnecting mam-millothalamic tract are comparatively small and thin. (2) In the medial group of the thalamus, the mediodorsal nucleus is remarkably large and merits special interest because of various connections with olfactory and limbic structures as well as a presumed phylogenetic size correlation with the frontal (orbital) lobe of the mammalian telencephalic hemisphere. (3) The ventral group consists mainly of somatosensory nuclei and constitutes a large part of the dorsal thalamus. In mammals generally, its ventral posterior nucleus (VPN) receives afferents via the medial lemniscus and the spinothalamic and trigeminothalamic tracts and dispatches a main projection to the somatosensory cortex. In the bottlenose dolphin, the ventral posterior nucleus is relatively small and projects to the neocortex anterior to the suprasylvian auditory area (Fig. 12). Compared to its lateral sub-nucleus, where the body region is represented, the medial sub-nucleus of the VPN with the head representation is relatively large. In dolphins, the limited somatosensory representation of the body is also reflected in the spinal cord (see later). (4) As in higher primates, the lateral group of thalamic nuclei in cetaceans is dominated by the massive pulvinar, the largest single complex in the thalamus of the bottlenose dolphin. The pulvinar more or less blends into both the strongly protruding medial geniculate nucleus (MG; auditory) and the large lateral geniculate nucleus (LG; visual). The main projection of the inferior pulvinar targets the suprasylvian gyrus, and that of the medial pulvinar die ectosylvian gyrus, whereas the lateral pulvinar projects to the border of the lateral and suprasylvian gyri. The MG is impressively large in cetaceans (Fig. 8) and reflects the outstanding development of the auditory system in these animals. Ventral portions of the MG project to the primary auditory area of the suprasylvian gyrus (Fig. 12) and dorsal portions to the "secondary" auditory area in the ectosylvian gyrus, as well as to the temporal operculum (perisylvian cortex). In the bottlenose dolphin, the LG is surprisingly well developed, although less so than the MG. The LG projects to the visually excitable part of the lateral gyrus (Fig. 12), but does not show the laminar organization usually associated with biretinal projection. This may be related to the fact that the fibers in die optic nerve show a complete or almost complete decussation in cetaceans.
3. Hypothalamus The basal part of the diencephalon exhibits an organization similar to that encountered in other mammals. Anterior, tuberal, and posterior hypothalamic nuclei are evident but not particularly prominent. Paraventricular and supraoptic nuclei are obvious because of their large hyperchro-matic cells, with the latter nucleus being especially well formed. As in other mammals the supraoptic commissure is well developed and well organized. The small size of the mamniillary bodies, which in the postnatal animal do not protrude at the brain surface, correlates with the weak development of the hippocampus, postcomniissural fornix, and mammillothalamic tract.
4. Hypophysis The pituitary gland in both toothed and baleen whales is rather wide (adenohypophysis), a pars intermedia and residual lumen are lacking. The neurohypophysis, which is separated from the adenohypophysis by a meningeal septum, is slender and consists of a pars nervosa, an infundibular stalk, and a median eminence. The adenohypophysis is composed of chromophobe, basophilic, and eosinophilic cells. The hypothalamohypophysial tract was reported to be wide in the bottlenose dolphin.
D. Brain Stem and Cerebellum
The percentage of the dolphin midbrain in the total brain volume is relatively low and ranges between that of prosimian and simian primates. Nevertheless, the size index, which is related to body size and the regression line of basal insectivores (shrews, hedgehogs, tenrecs), shows a remarkable increase of this structure even in plesiomorphic "river dolphins" (francis-cana; Ganges river dolphin). This may be attributable to the growth of auditory system components. The cerebellum and pons are well developed, and the myelencephalon (medulla oblongata) is very large in comparison with that of other mammals. This may be due to the considerable growth of cranial nerve nuclei and their connectivity, particularly those of the trigeminal, auditory, and motor systems.
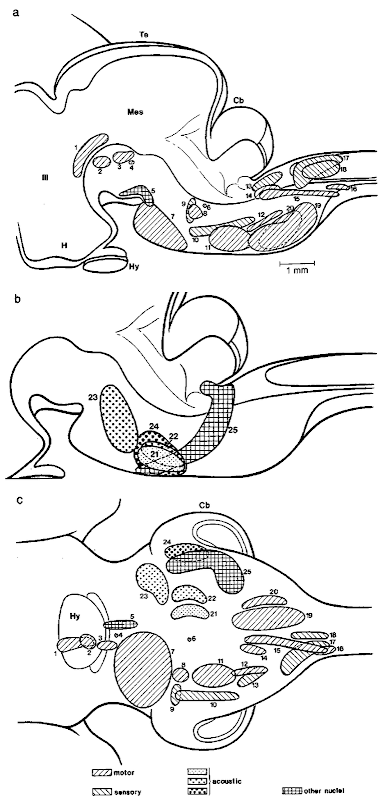
1. Selected Nuclei The cetacean brain stem comprises nuclei known from other mammals; some of them, however, are rather exotic and their functional properties are virtually unknown. Perhaps because they have changed considerably in appearance and/or location during evolution, a few nuclei have been described as unique to cetaceans so that they cannot be homologized easily with potentially corresponding nuclei in other mammals. Many of the features, however, presented in the literature on the cetacean brain stem could be confirmed in the work of Holzmann (1991) on the fetal narwhal (Fig. 11).
The oculomotor nucleus is the largest eye muscle nucleus, a fact that correlates well with the diameter of the oculomotor nerve. In comparison, the trochlear and abducent nuclei and nerves are rather small and thin in most cetaceans. The trigeminal nuclei (motor, principal, spinal nucleus) are very well developed, reflecting the large relative size of the cetacean head and die diameter of the trigeminal nerve, which is maximal or sub-maximal among the cranial nerves. Within cetaceans, the motor nucleus is reported to be larger in mysticetes but subdivided much better in odontocetes. Also the sensory principal nucleus is reported to be larger in baleen than in toothed whales, with its dorsal part giving rise to the well-developed trigeminothalamic tract (Wallenberg). The facial nucleus is very large in cetaceans and often bulges at the ventral surface of the medulla (tubercu-lum faciale). The nucleus is divided into a number of cell groups that can be differentiated from each other cytologically. These cell groups are believed to be responsible for specific muscles or muscular systems, e.g., the dorsal group for muscles of the upper respiratory tract around the blowhole (epicranial complex; Cran-ford et al, 1996), which are involved in the generation and emission of sonar signals in toothed whales. In comparison with other mammals, the ambiguus nucleus is large in cetaceans, which recalls to mind the situation in bats (mouse-eared bat; Mijotis mtjotis). This nucleus, which is larger in mysticetes dian in odontocetes, innervates the muscles of the pharynx, larynx, and die striated muscles of the esophagus via the glossopharyngeus-vagus-accessorius nerve complex. Comparable to other mammals, it should be involved in cetaceans in respiration, food processing, and sound production in the larynx. The nucleus of the accessory nerve, as part of the anterior horn in die spinal cord, is moderately well developed. This may be related to the extreme foreshortening of the cervical region, restrictions in head and shoulder girdle motion, and the transformation of the forelimb into a steering device (flipper). The hypoglossal nucleus, a derivative of the ventral horn of the spinal cord, is well developed, although the flexibility of the tongue is reported to be restricted in most cetaceans. In large baleen whales, the tongue may attain the body mass of a full-size elephant.
Nuclei related to or belonging to the extrapyramidal motor system are located in the rostral mesencephalon and in the for-matio reticularis throughout the rhombencephalon. The elliptic nucleus, which is situated within the central gray either rostral or dorsal to the oculomotor nuclear complex, is very conspicuous and in the past was thought to be unique for the Cetacea until a similar nucleus was found in the elephant. Moreover, it was unclear whether the nucleus of Darkscheivitsch is integrated into the elliptic nucleus or is even equivalent to this nucleus. Today, the latter opinion is the generally accepted one; In cetaceans, the elliptic nucleus projects via the medial tegmental tract to the rostral medial accessory inferior olive and correlates with a hypertrophy in cerebellar structures (see later). The red nucleus in cetaceans is little known. In contrast to ungulates, which possess a large rubrospinal tract and lack a spinal pyramidal tract, cetaceans have both weak rubrospinal and corticospinal tracts (see later). Pontine nuclei are exceptionally well developed in cetaceans, giving rise to the bilaterally ascending large brachia pontis, and the transverse pontine bundles are very numerous. The size and the caudal extent of the pons are directly related to the size of the neocortex.
The cetacean inferior olive is characterized by an extraordinary development of the medial accessory olive, particularly its rostral portion; in comparison, the principal olive and the dorsal accessory olive appear small. In the two cetacean suborders, there are only minor differences in the relative development of the subnuclei, and both inferior olives join each other in the midline. The rostral part of the medial accessory olive receives massive input from the elliptic nucleus via the medial tegmental tract, and its pronounced development in cetaceans seems to be related to the immense size of the posterior interposed nucleus and of the paraflocculus. In terrestrial mammals, the medial accessory olive is part of a fiber system involved in directional hearing.
2. Cerebellum The cetacean cerebellum is very large (Figs. 2-4, 6, 7, 9, and 10), its size obviously being linked phylogenet-ically with that of the neocortex. In older studies, the relative mass of the cerebellum in baleen whales with respect to total brain mass (average: 20%) was reported to represent a maximal development within the mammalia as a whole. A recent study has shown that in relation to body mass the cerebellum of baleen whales is not as voluminous as in larger dolphins such as the killer whale. Concomitantly, it became obvious that the large proportion of the cerebellum in the total brain volume of baleen whales is attributable to the relatively small size of the forebrain. Indeed, in double-logarithmic regressions, baleen whales rank a little higher than sperm whales, beaked whales, and "river" dolphins but distinctly below the delphinid cetaceans. With respect to the regression line in basal insectivores (Stephan), the cerebellum of the plesiomorphic La Plata dolphin ranks higher than the averages of prosimian and simian monkeys but lower than humans. In a group of delphinid species, indices of the total brain mass and cerebellum mass relative to body mass exceeded other groups (sperm whales, river dolphins, baleen whales) by up to three times. Within cetaceans, the cerebellum of the baleen whales is much better understood due to ontogenetic histological studies by Jansen (1950). Only minor structural differences exist, however, between the cerebella of toothed and baleen whales: Thus, the mysticete cerebellum is more rounded and slightly hourglass shaped in the dorsal aspect, whereas the odontocete cerebellum is somewhat more flattened dorsoventrally as a consequence of the stronger telescoping of the brain and resultant overlapping of the cerebellum by the cerebral hemispheres.
The cerebellum consists of two large hemispheres and a comparatively narrow vermis in between (Figs. 10 and 12). Two transverse fissures separate three cerebellar lobes: the primary fissure separates the small anterior (rostral third) from the large posterior lobe (caudal two-thirds), and the posterolateral fissure the posterior lobe from the small flocculonodular lobe. These size relations between the lobes are characteristic for cetaceans. In midsagittal section (Fig. 10), the conventional subdivision of the vermis into nine lobules of the mammalian cerebellum is obvious. In cetacean cerebellar hemispheres the small size of the anterior lobe may be explained by electrophysiological findings indicating that the hemispheral parts of this lobe comprise the cortical representation of the fore- and hindlimbs (cf. Jansen, 1950) that are highly modified or even have vanished in these animals. The caudally adjacent ansiform lobule, which also receives input from the extremities, is similarly small. However, the representation of the head in the simple lobule of the posterior lobe is rather large, and the considerable size of the paramedian lobule (body representation) has been related to the enormous significance of the tail in cetaceans. The paraflocculus, situated between the parafloccu-lar and posterolateral fissures, is exceptionally large, particularly the ventral parafloccular lobule (Fig. 3). The latter comprises about half of the surface of the cerebellar hemisphere. In mammals, generally speaking, the paraflocculus usually receives climbing fibers from the rostral part of the medial accessory inferior olive. In cetaceans, both structures are exceptionally large, which strongly indicates a functional relationship between the paraflocculus on the one hand and trunk and tail on the other (Jansen, 1950). The flocculonodular lobe as the principal terminus of primary and secondary vestibulocerebellar and visceromotor fibers ("vestibulocerebellum") is very small in cetaceans, particularly the floccular component (Fig. 10). In mammals generally, the latter is responsible for the regulation of compensatory vestibulo-ocular and optokinetic movements as well as compensatory movements of the neck in the so-called "smooth pursuit" movements of the eyes, particularly in carnivorous animals that lure and hunt. Dolphins, however, which have a restricted neck mobility and can use their visual system only during daylight, may have to rely on their auditory system instead to follow their prey effectively.
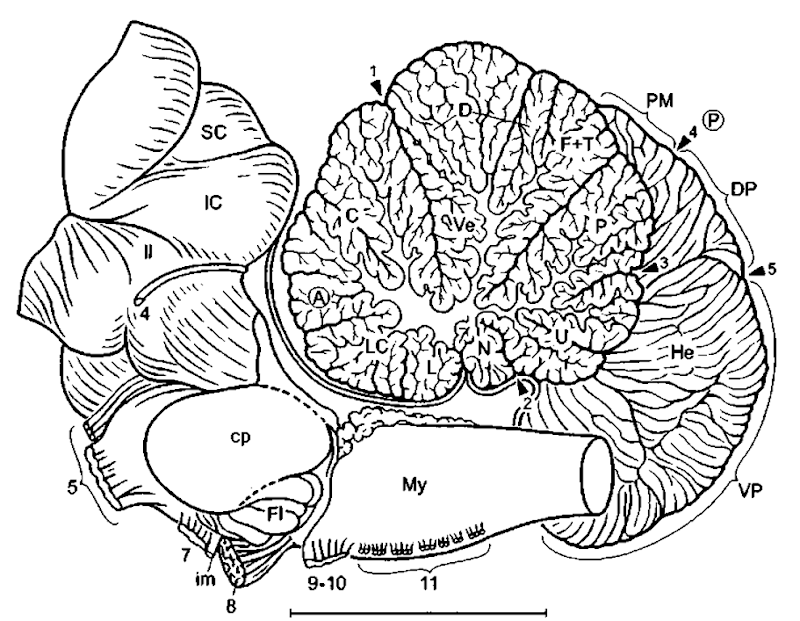
Figure 10 Brain stem of the fin ivhale (mediosagittal aspect): 1, primary fissure; 2, posterolateral fissure; 3, secondary fissure; 4, parafloccular fissure; 5, intra-parafloccular fissure; (A), anterior lobe of cerebellum; C, admen, D, declive; DP, dorsal paraflocculus; Fl, flocctdus; F+T, folium and tuber; He, cerebellar hemisphere; L, lingula; LC, lobus centralis; 11, lateral lemniscus; N, nodulus; (P), posterior lobe of cerebellum; P, pyramis; PM, paramedian lobule; U, uvtda. Modified after Jansen (1953). Scale: 1 cm.
In the ontogenesis of the cetacean cerebellar cortex, which is three layered, the fundamental mammalian pattern of transverse and longitudinal zones is discernible. These longitudinal zones obviously are related topographically to the development of the cerebellar nuclei (anterior, medial, and posterior interposed nuclei, lateral cerebellar nucleus). In cetaceans, the lateral intermediate cortical zone (C2 zone) is developed enormously, occupying about three-fourths of the cerebellar surface (paraflocculus) and correlating with the huge posterior interposed nucleus.
3. Main Fiber Systems Medial lemniscus: In cetaceans, the afferent spinal system (proprioceptive sensitivity) is moderately developed in accordance with the reduction of the hindlimbs and pelvic girdle. In these animals, the dorsal funiculi (gracile and cuneate fascicles) are strikingly small; they are thought to convey input predominantly from the flippers and the tail (sense of position). Nevertheless, cutaneous sensitivity in the trunk was reported to be high (Slijper, 1962). The medial lemniscus is weak in the caudal medulla, but becomes considerably stronger at more rostral medullary levels, presumably due to the input of afferent systems of the head (auditory, trigeminal systems).
Trigeminothalamic tract (Wallenberg): The dorsal part of the principal sensory trigeminal nucleus gives rise to the ipsi-lateral (uncrossed) trigeminothalamic tract. The latter terminates in the medial part of the ventral posterior thalamic nucleus as the main somatosensory thalamic nucleus. As in ungulates and the elephant where it is extremely well developed, the tract is thought to be responsible for intra- and perioral sensitivity in cetaceans innervating, e.g., tactile bodies on the hps of fin whales and sei whales (Balaenoptera borealis), and the epicranial complex (Cranford et al., 1996) in toothed whales.
Figure 11 Brain stem of fetal narwhal (Monodon monoceros) with major nuclei (from Holzmann, 1991, modified). The physiological quality of the nuclei is indicated by different textures, (a) Mediosagittal Wiped, (b) detail of (a) with auditory nuclei, and (c) synopsis of all nuclei in basal aspect. Mes, mesencephalon; 1, elliptic nucleus; 2, interstitial nucletis; 3, nucleus of oculomotor nerve; 4, nucleus of trochlear nerve; 5, interpeduncular nucleus; 6, nucleus of abducens nerve; 7, pontine nuclei; 8, motor nucleus of trigeminal; 9, principal nucleus of trigeminal; 10, spinal nucleus of trigeminal; 11, nucleus nervi facialis; 12, nucleus ambiguus; 13, nuclei of solitary tract, 14, dorsal nucleus of vagus; 15, nucleus of hypoglossal nerve; 16, spinal nucleus of accessory nerve; 17, cuneate nucleus; 18, gracile nucletis; 19, medial accessory inferior olivary nucleus; 20, principal and dorsal accessory inferior olivary nuclei; 21, nuclei of trapezoid body; 22, superior olivary complex, 23, nuclei of lateral lemniscus; 24, ventral cochlear nucleus; 25, pontobulbar nucleus. Scale: 1 mm.
Figure 12 Neocortical motor and sensory fields in the bottlenose dolphin. Al, A2, auditory fields; crs, cruciate sulcus; M, motor field; PL, paralimbic lobe; S, somatosensory field; V, visual field. After Morgane et al. (1986).
Medial tegmental tract: The elliptic nucleus (Figs. 2b and 11), which almost “replaces” the red nucleus in Cetacea, is developed enormously in whales and elephants and gives rise to the strong medial tegmental tract that proceeds to the rostral part of the huge medial accessory inferior olive. This nucleus also receives afferents from the spinal cord (spino-olivary tract) and projects to the lateral intermediate (C2) zone in the huge paraflocculus. The paraflocculus, which was shown to be the main target of auditory pontocerebellar projections in the rat and was estimated to receive three-fifths of the pontocerebellar fibers in the blue whale (Balaenoptera musculus), has a massive projection to the posterior interposed nucleus of the cerebellum. From the latter nucleus, ascending fibers run to the elliptic nucleus and other nuclei at the diencephalic/mesencephalic border, which, in turn, project to the inferior olive. Like the medial accessory olive, the paraflocculus also has been associated with mass movements of the trunk-and-tail region, the only region where the axial skeleton possesses a reasonable range of motion. The medial tegmental tract thus seems to be part of a recurrent circuit (elliptic nucleus-inferior olive-paraflocculus-posterior interposed nucleus-elliptic nucleus), which combines auditory input with locomotor activity and illustrates the dominant position of hearing among sensory systems in whales.
Pyramidal tract: The tract originates in the neocortical motor area rostral and medial to the “cruciate” sulcus (Figs. 2a and 12) and runs through the internal capsule. At mesencephalic levels, the localization of the pyramidal tract is difficult and it is very small at high medullary levels. Typical macroscopical “pyramids” as seen in terrestrial mammals are not present in cetaceans. Here, the pyramidal tracts are weak and situated lateral to the inferior olivary complex, with both inferior olives joining each other midsagittally (Figs. 3, 7, and 9). Obviously, the extremely well-developed rostral medial accessory olive, which occupies the ventromedial area in the medulla, has pushed the pyramids lateralward. Caudal to the inferior olives, the pyramidal tract disappears in baleen whales. In toothed whales, both pyramids merge with each other; their crossing (decussation) is described by most authors as indistinct and the pyramidal tract as small and hardly visible. Thus, it is likely that pyramidal (corticospinal) fibers do not descend more than a few (cervical) segments in the spinal cord. This pattern resembles much that is seen in hoofed animals and the elephant. In terrestrial mammals, an inverse relationship exists between the development of the pyramidal tract and of the rubrospinal tract. Perissodactyls and artiodactyls have small pyramidal tracts, whereas their rubrospinal tracts are large. The opposite is seen in primates whose small rubrospinal tract is coexistent with a large spinal pyramid. Such an inverse relationship is not encountered in Cetacea: Here, both the rubrospinal and corticospinal tracts are small, obviously a speciality in cetaceans.
II. The Cetacean Spinal Cord
Within the nervous system, the spinal cord (Figs. 4a and 8) is responsible for the innervation of the trunk and tail, and the pectoral and pelvic girdles together with their appendages. Thus, the spinal cord mediates between the locomotory apparatus of the body and the brain by transmitting sensory vs motor information.
All cetaceans are characterized by (1) the subtotal reduction of the pelvic girdle and hindlimb and the transformation of the pectoral girdle and forelimb into a steering device and (2) the extraordinary development of the axial musculature, thus contributing to the spindle shape of the body required for efficient locomotion by the trunk-and-tail complex.
With respect to the sensory innervation of the body in whales and dolphins, we do not know anything about the innervation of joints and tendons. The skin of the body is hairless. Nevertheless, the assumption of poor sensory innervation of the skin in cetaceans has been contradicted by Slijper (1962), who observed reactions of stranded and captive dolphins to the gentlest touch.
In correlation with the large amount of axial musculature, the motor ventral roots of the cetacean spinal nerves appear to be larger than the dorsal ones, particularly in the posterior part of the system (cauda equina). There are up to 97 pairs of spinal nerves (e.g., 44 in the harbor porpoise: 8 cervical, 11 thoracic, and 25 lumbocaudal). On each side of the body, along the vertebral column, there are two plexus (cervical plexus: C]-C3; brachial plexus: C4-Th]). The peripheral nerves supplying the tail can be very long (up to 15 m in large baleen whales).
Other remarkable features of the cetacean spinal cord are its relative length and shape. Whereas in the harbor porpoise the relation of body length to spinal cord length is 3.75:1, baleen whales have values from 4:1 (fin whale) to 6.7:1 (right whale; Eubalaena sp.). A cervical enlargement (intumescence) for the innervation of the pectoral girdle and flipper has been described in different odontocetes and in the fin whale. The presence of a caudal intumescence, however, is controversial.
The internal structure of the spinal cord reflects peculiarities of the cetacean motor and sensory systems: long and slender anterior horns, occupying half of the gray matter, the posterior horns modest to stunted in appearance (Fig. 8a). A short and pointed lateral horn is discernible at thoracolumbar levels. The gelatinous substance, in general, is remarkably poorly developed in cetaceans, with only traces found in some spinal segments of the harbor porpoise and the fin whale. In mammals, this crescentic mass of small interneurons is involved intimately in the connectivity of cutaneous afferents of the dorsal spinal root with delicate fibers of the spinothalamic tract (pain and temperature). In cetaceans, the lack of gelatinous substance may correlate with the loss and modification of the posterior and anterior limb, respectively.
Whereas cell density and numbers of neurons in the cetacean spinal cord are not known, the dimensions of the cells in the anterior horn have been investigated in baleen whales. Despite the large difference in body mass, the sizes of the perikarya in the cervical and thoracic spinal cord are similar in the whale and human, but in the lumbocaudal area the whale has much larger perikarya with a volume presumably several times larger than in comparable human perikarya.
III. Functional Systems A. Chemoreceptor Systems
Adult baleen whales still have a small, but functional, nose probably equipped with olfactory mucosa and an olfactory nerve, and they possess an olfactory bulb, slender olfactory peduncle, and an olfactory tubercle (Figs. 3 and 7), a situation resembling that of the human. Presumably as a consequence of the adaptation to sonar orientation, prey detection, and communication (high-energy sonar clicks in the upper respiratory tract), toothed whales have reorganized their nasal system to such a degree that (at most) a short olfactory peduncle is found in the adult animal very rarely [sperm whale; northern bottlenosed whale (Hijperoodon ampullatus)]. In general, toothed whale embryos display the anlage of an olfactory bulb, but the latter is reduced in early fetal stages. Interestingly, however, a “residue” of the bulb persists in the large ganglion of the terminal nerve (Buhl and Oelschlager, 1986) that is responsible for the establishment of the hypothalamo-hypophyseal-gonadal axis in prenatal mammals, and thus for sexual behavior and reproduction in adults. In dolphins, the terminal ganglia contain the highest number of neurons found within the mammalia (Ridgway et al., 1987). These facts argue both for the nonolfactory nature of the terminal nerve and its possible implications in the control of blood flow in the nose and basal forebrain, in maintenance of the epithelial lining of the upper respiratory tract, and in the sensory control of sonar signal emission as well. A vomeronasal organ (Jacobson’s organ) and nerve are absent in cetaceans and sirenians.
Despite the strong general reduction of the olfactory system in toothed whales, the olfactory tubercle seems to be well developed even in comparison with that in baleen whales, which possess a small but presumably functional olfactory system. In many cases, the tubercle may not be separated clearly from the neighboring diagonal band and prepiriform cortex, a configuration often called the “olfactory lobe” (Fig. 2a). In reality, it is the large corpus striatum (basal ganglia) that protrudes here at the basal surface of the brain as an “olfactory” tubercle and is covered by an incomplete layer of thin paleocortex analogous to the situation in humans. The remaining paleocortex (diagonal band, piriform cortex) is moderately developed. The amygdala as a whole, however, is rather large in cetaceans for other reasons.
Dolphins are clearly sensitive to chemical stimuli (both natural and artificial compounds), and some cetaceans were reported to have functional taste buds. With the olfactory part of the nose disappearing during prenatal development, dolphins still may resort to their taste buds and the trigeminal innervation of the oral cavity for chemoreception.
B. Visual System
In most cetaceans, the visual system is reported to be fairly well developed (Figs. 2-4, 6-8, 10, and 12). In the adult harbor porpoise, the optic nerve contains 81,700 axons, 147,000-390,000 axons in the bottlenose dolphin, 193,000-250,000 axons in the domestic cat, and 1,200,000 in the human. The Amazon river dolphin (Inia geoffrensis) shows a rather low axon count (15,500), and the optic nerve of the Ganges river dolphin, whose eye lacks a lens and may be capable of serving as a light receptor only, contains a few hundred axons. Large whales have moderate numbers of axons. In baleen whales, the optic nerve contains approximately the same number of axons (252,000-347,000) as in the bottlenose dolphin, whereas the sperm whale, with roughly the same body weight and relatively smaller eyes, has only 172,000 axons.
Bottlenose dolphins have a thick retina composed of rods and cones. The fovea centralis is band shaped, and neurons in the ganglionic layer are very large (up to 150 |xm in diameter) with thick dendrites and myelinated axons of up to 9 |xm thick. Cetaceans have laterally placed eyes and, if at all, one small binocular visual field rostrally and ventrally as well as another one dorsally and slightly caudally. The optic fibers show a complete or almost complete decussation, and the lateral geniculate body is not laminated. The superior colliculus is large in most cetaceans, as is the case in many land mammals, including carnivores, ungulates, and primates. Dolphins show a definite stratification of the superior colliculi, where layers typical of terrestrial mammals are recognizable. In some baleen whale species, the superior and inferior colliculi are approximately the same size (length and width), whereas in others [Southern right whale (.Eubalaena australis); blue whale {Balaenoptera musculus)] the inferior colliculi have double the surface of the superior colliculi. In toothed whales, this relation may range from 2:1 up to 7:1 (maximum: susu).
Electrophysiological mapping studies in dolphins have located the visual cortex not in the dorsocaudal or occipital part of the hemisphere but in a more central position near the midline (Fig. 12). Although well developed, the physiological importance of the cetacean visual system theoretically should be inferior to that of the auditory system because of functional restrictions in murky water and in darkness.
C. Auditory System
The auditory system in whales and dolphins basically corresponds to that in terrestrial mammals; however, the various components have been adapted morphologically and physiologically to the specific conditions of hearing under water (Figs. 2, 3, 4a. and 5-12).
In general, auditory structures are smaller in baleen whales than in toothed whales (compare Figs. 3 and 7-10). According to the impressive diameter of the vestibulocochlear nerve and the number of axons in the cochlear nerve, the cetacean ventral cochlear nucleus (VCN) and other auditory centers are very large (Figs. 5, 11, and 12). The secondary auditory fiber tracts (trapezoid body, acoustic striae) are well developed. In the La Plata dolphin as well as in the harbor porpoise and the common dolphin (Delphinus delphis), the absolute volume of the cochlear nuclei is 6-10 times larger than in the cat and the human; in the beluga (Delphinapterus leucas) it is 32 times larger, and in the fin whale it is 20 times larger. Numbers of cochlear neurons range between 583,000 and 1,650,000, i.e., 6-17 times that of the human. The ratio of primary cochlear nerve fibers to secondary cochlear neurons is between 1:5 and 1:8 (human: 1:4). The volume of other auditory nuclei is either very large in comparison or very low, even if we take into account the different body mass of the animals. In the La Plata dolphin, multiples 17X the volume of the cat’s nuclei are found in the nucleus of the trapezoid body and 39X in the intermediate nucleus of the lateral lemniscus. A comparison between volumes of auditory nuclei in the common dolphin and the human yields similar results. There are multiples for the dolphin VCN (16X), lateral superior olive (150X), single nuclei of the lateral lemniscus (up to 200 X), and for superior auditory centers such as the inferior colliculus (12X) and laminated medial geniculate nucleus (7X). The cetacean VCN is composed of five subunits consisting of specific neuron populations that are also found in terrestrial mammals. This has been proved by cy-tological investigations in the franciscana (Schulmeyer, 1992) involving the morphology of the axon terminations. The nucleus of the trapezoid body is well developed: A great mass of fibers passes from the VCN into the trapezoid body, to the ip-silateral and contralateral nuclei of the lateral lemniscus, and the inferior colliculus. Interestingly, the dorsal cochlear nucleus (DCN) could not be found in many toothed and baleen whales, perhaps because of its reduction to the point of insignificance. Obviously, this nucleus is engaged in the assessment and/or elimination of “auditory artifacts” caused by positional changes of the head and pinnae toward a sound source. In terrestrial mammals (including bats) with “normal” external ears and good movability of the head and pinnae, the DCN is well developed and even laminated.
The existence of the medial superior olive (MSO) is discussed in the literature on toothed whales. In some species, the two subnuclei of the superior olive cannot be distinguished, and whether the medial nucleus is very small or even lacking in other species, as has been reported for bats, is not clear at present. In mammals, the MSO is believed to be engaged in the processing of low frequencies and the lateral superior olive (LSO) in the processing of high frequencies and ultrasound. Therefore, it has to be expected that the LSO is larger in toothed whales and the MSO in baleen whales, reflecting the actual neurophysiological adaptation (audiogram) of these animals. The components of the midbrain tectum differ in size in baleen whales and toothed whales. Whereas in mysticetes the superior colliculi (vision) may be larger or smaller than the inferior colliculi (audition), odontocetes always have very large inferior colliculi (Figs. 2b, 4a, 5, 6, 8, and 10). The striking dominance of the sense of hearing in adult toothed whales has been emphasized by a number of investigators, among them Jansen and Jansen (1969), Morgane and Jacobs (1972), Schulmeyer (1992), and Oelschlager and Kemp (1998). Indeed, the auditory system has stamped the morphology, size, and connectivity of the whole odontocete brain due to the necessity of processing vast amounts of acoustic information and on account of the high propagation velocity of sound in water (Ridg-way, 1986, 1990) and the need of background noise discrimination. In the adult sperm whale and other toothed whales, the auditory system seems to be the major source of information. Even when the animals dive to considerable depths and the visual system progressively loses its importance for orientation and the detection of prey, the auditory system may still be functional (navigation by auditory input). Toothed whales scan their surroundings with a sonar system (clicks) and are probably able to integrate the acoustic input together with visual in formation into two- or even three-dimensional ephemeral images within their extended neocortical auditory projection fields (Supin et al., 1978). In addition, communication between individuals by means of acoustic signals clearly is very important for whales and dolphins, especially during hunting activity and/or when vision is reduced. Pelagic dolphins, in particular, tend to live in larger groups. In the open sea, their natural environment is largely represented by the distributional pattern of their kin, which changes more or less continually.
D. Vestibular System
The four major vestibular nuclei usually found in mammals are also present in cetaceans. The lateral vestibular nucleus of Deiters in cetaceans is most conspicuous and its dimensions fairly large, but the number of neurons does not seem to be exceptional. The other nuclei are rather small. The lateral vestibular nucleus does not receive projections via the vestibular nerve, and its connections to the lateral B-zone of vermis, dentate nucleus, fastigial nucleus, and the oculomotor complex are very similar to those of cerebellar nuclei. In mammals generally, the other vestibular nuclei receive input from the semicircular canals, but these are minute in cetaceans. All of the vestibular nuclei project via the medial vestibulospinal tract and medial longitudinal fasciculus to motor neurons of the neck musculature and to the nuclei of the ocular muscles, and they are involved in reflex arcs harmonizing the position and precision movements of the head with those of the eyes in the pursuit of prey.
E. Limbic System
In odontocetes. the various cortical and subcortical components of the limbic system show different degrees of development (Figs. 2b and 3-7). In adult toothed whales, including the sperm whale, the hippocampus and mammillary body are unusually small and interconnected by a relatively thin fornix, whereas the anterior thalamic nuclei and the habenulae are better developed as in other mammals. The amygdaloid complex is large in toothed whales (Schwerdtfeger et al, 1984). In contrast to the nucleus of the olfactory tract, which is totally dependent on olfactory input, the amygdala (taken as a whole) is well developed in microsmatic species (baleen whales, human) and even in anosmatic species (toothed whales). This indicates that, apart from olfactory stimuli, input to the amygdala arises from other sources, among them the auditory system. Furthermore, the remarkable development of the cortical limbic lobe (periarchicortex) in the dolphin again points to the largely nonolfactory character of this system.
F. Characteristics of Major Peripheral Cranial Nerves
The eye muscle nerves are thin in small cetaceans, particularly in river dolphins with their reduced eyes where they may even be completely lacking (Pilleri and Gihr, 1970). In marine dolphins, the oculomotor nerve comprises the highest number of axons (about one-third that in the human), followed by either the trochlear or abducent nerves. In large whales (especially baleen whales), the numbers of axons in the eye muscle nerves are distinctly higher and correspond to the situation in humans.
The trigeminal nerve is the thickest cranial nerve in baleen whales and sometimes in the sperm whale as well, whereas in all of the other toothed whales the vestibulocochlear nerve has the maximal diameter (Figs. 2a, 3, and 7-9). The diameters of the axons tend to be thin in the trigeminal nerve, whereas cochlear axons rank among the thickest axons known. In the smaller toothed whales investigated, the trigeminal nerve contains 82,000-156,000 axons (human: 140,000). The fact that trigeminal nerves in sperm and baleen whales have about the same large number of axons (490,000 vs 370,000-500,000) may be explained by the extreme size of the forehead region innervated in these animals.
The vestibulocochlear complex is the largest cranial nerve in toothed whales, and occasionally in the sperm whale as well, and it is the second largest in baleen whales (Figs. 2a, 3, 5a, and 7-10). In the sperm whale, it contains 215,000 axons, whereas in baleen whales, axon numbers vary from 154,000 to 179,000. Smaller toothed whales have 84,000 (harbor porpoise) to 171,000 axons (beluga), with the latter thus ranking near the much larger baleen whales (human: 50,000). The ratio of auditory and vestibular axons within the eighth nerve is disputed in the literature. Whereas some authors found that cochlear fibers in the bottlenose dolphin (total: 116,500 fibers) comprise about 60% of all the vestibulocochlear fibers (a percentage known from the human), other authors reported a similar total axon number (113,000) but a much higher fraction of cochlear axons (97%) as opposed to a very low number of vestibular axons. In comparison with humans (19,000 vestibular axons), dolphins possess only one-fifth in vestibular axons, a fact that correlates well with their small semicircular canals, with their low number of vestibular ganglion cells, and with the observation that the diameter of the vestibular nerve is barely one-tenth that of the cochlear nerve.
The variation in thickness of the facial nerve and in the diameter of its fibers throughout the cetaceans again sheds some light oil biological correlations. Thus, the facial nerve in the sperm whale is nearly as thick as the vestibulocochlear nerve, containing about three times as many axons as in large baleen whales and three to eight times more axons than in smaller toothed whales. Baleen whales rival sperm whales in body size, and in all of these giants the head may attain one-third of the total length and mass of the body. In contrast, the absolute and relative size of the head in other toothed whales is comparatively small. Accordingly, it may be speculated that the prominent thickness of the facial nerve and the large number of its motor axons in the sperm whale are attributable to the extreme size of the forehead, which has to be regarded as an oversized “sound machine” (Cranford). Here, the forehead is characterized by the unique amount of acoustic fat tissues and massive blowhole musculature (innervated by the facial nerve) that may help stabilize the giant fat bodies and adjust their shape (acoustic lenses) during the emission of sonar signals.
IV. Neocortex
A. Layering and Cell Morphology
For a biological interpretation of the cetacean brain, we must review some existing data about the neocortex, e.g., brain/body mass relationship, absolute and relative cortex mass, and the structure of the neocortex, i.e., the layering, neuron density, synapse number and density, as well as density of gliocytes. The latter cells nourish, isolate, protect, and possibly help stimulate and regenerate the neurons and therefore are as essential for neocortical activity as the neurons themselves.
No other brain surpasses the cetacean brain in richness and complexity of neocortex gyrification. Moreover, toothed whales exhibit encephalization indices similar to those of higher primates. The cetacean neocortex shows an extremely tight folding and has a maximal surface within mammalia. At the same time, dolphins have cortical widths known from ungulates (Hummel, 1975), and even large whales do not seem to attain the average cortical thickness of higher primates (human).
In principle, the cetacean neocortex is six layered and similar to that in other mammals (Fig. 5b). Regional differentiation, however, is less obvious, and cortex lamination is not well expressed, particularly due to the widespread absence of a distinct layer IV in adult toothed whales and the moderate gran-ularization of the cortex in general. In whales, layer one (molecular layer) can comprise up to about one-third of the total cortical width, whereas layer II is thin but rich in small pyramidal neurons that stain intensively with cresyl violet (Nissl stain). This second layer corresponds to the outer granular layer in the human. Its pyramidal cells that border on the molecular layer are mostly “extraverted” in whales, i.e., they show a clear predominance of apical (subpial) dendrites over basal dendrites. Layer III is thick and characterized by a variety of pyramidal cells with comparatively smaller neurons populating the outer half of the layer, whereas larger cells occupy the inner half. In view of the special role played by laver IV (corresponding to the inner granular layer in the human) as a major input layer for thalamocortical specific afferents in advanced land mammals, the precise cytoarchitectonic definition of this layer in cetaceans and its neuronal composition are crucial. It was postulated that in eutherian mammals layer IV appears and develops in proportion to the progressive displacement of specific thalamic afferents from the first (molecular) layer to mid-levels of the cortex. In evolutionary advanced primates and ungulates, layer IV of the sensory neocortex is relatively wide and consists mainly of small granular, i.e., nonpyrainidal cells mostly of the spiny stellate type. The fact that a lower degree of gran-ularization also exists in insectivores and insectivorous bats and that layer IV is often not discernible here led to the assumption that the structure of the cetacean neocortex is “primitive” and allegedly shows a considerable number of other plesiomorphic features. These are the lack of definite boundaries, gradual morphological transitions between functionally separate neocortical areas, a poor lamination pattern, a thick layer I, extraverted pyramidal neurons in layer II, a dense band of large pyramidal neurons referred to as layer 111,7V, overall weak granularization with a predominance among nonpyramidal neurons on the part of large isodendritic stellate cells, a well-developed layer VI, and a lack of giant pyramidal cells. In some cortical areas of the fin whale brain, however, a narrow zone where nerve cells are sparse or lacking seems to mark the site of layer IV, and the corresponding external stria of Baillarger was found in a dolphin. Very young postnatal bottlenose dolphins also show a “remnant” of this layer. The innermost part of the cetacean cortex, presumably corresponding to layers V and VI in terrestrial mammals, usually displays no clear stratification and fades out into the white matter.
According to Deacon (1990), the common ancestor of all cetaceans very likely possessed layer IV granule cells as do the hyraxes or conies, plesiomorphic members of the paenungulate order (animals with precursors of genuine hooves), which includes the sirenians and elephants. In the highly encephalized elephants, layer IV was found to be lacking in all cortical areas, and their neocortex architecture is strikingly similar to that of the fin whale. The loss of granule cells in layer IV in cetaceans was therefore regarded a rare derived trait that may be correlated with the shift of the orphaned terminations of the specific thalamic afferents (originally terminating in layer IV) back to layers I and II, thus allowing more neurons in layer II to persist.
However, based on Golgi studies, there is little reason for thinking that the neocortex in the dolphin exhibits less variety than in other mammals, and a fair proportion of true stellate and other nonpyramidal cells exists in the dolphin cortex scattered throughout layers III and V, which may combine functions of afferent, efferent, and associative layers. Moreover, the distribution of neuronal size classes is rather similar to that occurring in other mammals.
Comparative iinmunocytochemistry (GABA, calcium-binding proteins) in the primary visual and auditory cortex of dolphins has revealed that the overall quantitative characteristics of the neurons are similar to those in other mammalian orders, being closer to insectivores and rodents in some features and to bats and primates in other characteristics.
B. Neuron Density
The density of cells is subject to considerable variation throughout the cetacean neocortex not only from area to area but also within the individual layers, although the relatively small and densely packed neurons in layer II account for at least 50% of all the neurons in a given cortical block. Tower (1954) measured the neuronal density in unspecified cortical areas of two fin whales, arriving at a mean figure of 6800 cells/min3. He also reported a similar neuronal density (6900/min3) in an elephant brain of about the same size. In comparison with approximately 18,000/mm3 in the human and more than 100,000/mm3 in rat and mouse, Tower concluded that there was a direct relationship between cortical neuronal density and brain mass. Other cell counts, however, reported an average of 57,000 neurons/mm3 for the adult human. Cell counts in a series of toothed whales again revealed some decrease in density with increasing body size and absolute brain mass (harbor porpoise, 13,200; bottlenose dolphin, 13,000-14,200; beluga, 12,300; humpback whale, 8300/mm3). Total counts of neurons beneath 1 mm2 of cortical surface do not take into consideration the different width of the neocortical plate in various mammals and result in 28,500 cells for the adult bottlenose dolphin and 46,400 in an animal of 18 days. By comparison, the figure of 147,000 was given for all neocortical areas studied in a series of mammals ranging from mouse to human, except for the primate visual cortex, which harbors about twice as many neurons. Cell counts in single cortical sampling columns from pial surface to white matter with a cross-sectional area of 25 X 30 (xni resulted in 19 cells for the elephant, 21 for the bottlenose dolphin (Giinturkun, personal communication), and 110 in the nonstriate cortex of other mammals. Although Rockel’s assumption may be restricted to primates, carnivores, and some rodents, dolphins obviously have a much smaller number of neurons in a given volume sample of cortex than would be expected from the size oi their brains.
The mean size of neuronal perikaiya has no definitive relation to brain size. Whereas primates tend to have a similar mean neuronal size and size distribution, other mammals, including cetaceans, show a tendency to increase neuronal size with increasing brain weight. Dolphins have moderate neuron sizes more or less similar to those found in ungulates (sheep, cow, horse) and elephants. Volume measurements reported a maximal neuronal volume of over 20,000 |xm3 in the harbor porpoise, which is about double the maximal volume found in ungulates and small to medium sized for the human.
C. Synapses
The synaptic parameters of the visual neocortex show many of the qualitative and quantitative features of the general mammalian bauplan. For example, the quantitative relationship between the number of synapses with contacts to different components of cortical neurons such as perikaryon, dendritic shafts, and dendritic spines in the dolphin cortex does not differ significantly from these parameters in most other mammals. The same holds true for the distribution of other synaptic parameters throughout the cortical layers such as the area and form factor of individual synaptic boutons, the length of active zones (densities) of synaptic membranes, and several parameters relating to synaptic vesicles.
In their neocortex, dolphins show a mixture of conservative and advanced features at the cortical architectonic level as well as at neuronal and synaptic levels. When looking at the cortex as a whole, the aforementioned so-called conservative features occur mostly in superficial layers I and II, whereas deeper layers III—VI are characterized as more or less equivalent to those in advanced terrestrial mammals with the exception of layer IV. To date, adequate comparative data on ungulate groups or other aquatic mammals are not available. Moreover, cetaceans have a much lower neuronal density but, at the same time, a much higher synaptic density per volume unit and per neuron than terrestrial mammals. The majority of all synapses (70%) is found in cortical layers I and II: The latter seem to receive the brunt of cortical input as opposed to layer IV in many terrestrial mammals. Thus, in cetaceans, layer II seems to be the main relay element conveying information from the subcortical and intracortical afferents via layer I to the other cortical layers. All of these data, however, are difficult to interpret as to their significance both for function and/or physiology and for the evolution of the cetacean neocortex.
With respect to the total number of synapses in their cortices (0.87 vs 1.3 X 1014), the dolphin and human resemble each other much more closely than other mammalian species. This appears to reflect primarily the generally large volume of neocortices in both dolphin and human brains as well as the maximal number of synapses per neuron compensating for minimal neuronal density in the dolphin.
D. Glia
Only a few data are available on gliocytes in the cetacean neocortex. In the bottlenose dolphin, glial density was found to vary in different cortical areas from 28,000 to 93,200 cells/mm3, values rather similar to those in the human (average: 40,000-100,000 cells/mm3). The number of gliocytes per number of neurons (glia/neuron ratio) is species-specific, i.e., varies among the mammalian groups and during ontogenesis due to changes in neuron density. This basically implies that larger brains have higher glia/neuron ratios. Thus, the ratio rises from small rodents (mouse, rabbit: 0.35) via ungulate species (pig, cow, and horse: 1.1), the human (1.68-1.78) and bottlenose dolphin (2-3.1), to large whales (fin whale: 4.54-5.85). Consequently, the glia/neuron ratio within each species increases from birth to maturity, thereby signaling the importance of glia for growing neurons and, thus, for neocortical function. Another implication is the necessity of comparing only mature specimens. Accordingly, in species with similar neocortex volumes, the glia/neuron ratio may be interpreted both ways, i.e., in favor of neurons or glia as factors of equal importance.
In summary, the morphology of the dolphin neocortex seems to be equivalent to that of advanced terrestrial mammals, but its specific features are not yet understood. With respect to ontogenesis and evolution, the allometric process of thinning out the number of neuroblasts and/or neurons via apoptosis and via the generation of increasing amounts of glia and neuropil for their connectivity seems to proceed faster in cetaceans than in other mammals. At the same time, the cortical plate seems to spread out more than in other mammals, leading to an extremely extended and convoluted neocortex with a minimal neuron density but maximal synaptic density.
V. Characteristics of Semiaquatic and Aquatic Mammals
A. General Remarks
The brain can be regarded as the true center of the body responsible for the maintenance of physiological conditions (homeostasis) and survival. By means of the cranial and spinal nerves, the brain collects all of the sensory information available, thereby evaluating, synthesizing, and using it for optimal behavior in manifold aspects: orientation, feeding, defense, communication, and reproduction. Thus, the brain not only represents all parts of the body but, at the same time, mirrors the situation of the animal within its ecological niche. On account of these tight correlations, evolutionary changes in the environment and in the biology of the species must show in the morphology of the nerves and brain. Rapid transgressions of mammalian groups into totally different habitats are related to strong “selection pressure,” i.e., adaptational processes unfold rather quickly and may lead to profound changes in brain morphology and function, reflecting changes in the body’s periphery. One obvious principle is seen in the increase or decrease in size of individual brain structures, which may result in qualitative changes. Comparative analysis of the brain, therefore, can help unveil evolutionary strategies of the species by looking for qualitative and quantitative correlations between various brain structures and body mass and between those structures belonging to the same functional system.
B. Insectivores
The most obvious changes in the quantitation of the brain in all semiaquatic insectivores are as follows: a marked reduction of the primary and secondary olfactory centers, a slight to moderate size increase of the neocortex and myelencephalon, and a moderate to marked growth of the diencephalon (dorsal and ventral thalamic nuclei, geniculate nuclei), as well as other structures related to the trigeminal, auditory, or motor systems. There is a general but weak size increase of the limbic system, with the hippocampus showing the least change among the components of this system in comparison to terrestrial relatives. The trigeminal complex is thought to replace the olfactory system as the main sensory system in the search for food (vibrissae), whereas the accessory olfactory (vomeronasal) system seems to be unaffected by the animal’s adaptation to water.
C. Carnivores
Other examples of convergent evolution are single mustelids (otters) and the pinnipeds. In comparison with their terrestrial relatives and with respect to basal insectivores, otters are distinctly more encephalized, and the same seems to be valid for pinnipeds (seals) and their potential terrestrial relatives. In contrast, sirenians have been reported to have low en-cephalization indices in comparison with herbivorous terrestrial “relatives,” such as the hyraxes, a fact that may be related to the comparatively high body mass of sea cows and the ar-boricolous mode of life in hyraxes.
1. Eurasian River Otter (Lutra lutra) Here, the adaptations to the aquatic medium are only moderate: The olfactory system is markedly smaller than in terrestrial carnivores but better developed than in the seal. The hippocampus and amygdala are large. The visual system is well developed (thick optic nerve, lateral geniculate nucleus layered). The same holds true for the auditory system, but die acoustic tubercle (dorsal cochlear nucleus) is small. The trigeminal system is well developed (vibrissae), but the facial nerve and nucleus are of moderate size. As in the seal, the basal ganglia are moderately developed, but the globus pallidus is large. In the cerebellum of the otter, the vermis is considerably smaller with respect to the hemispheres than in terrestrial mammals but larger than in pinnipeds. The inferior olive in the otter is organized similarly to that in terrestrial mammals, but it is larger than in these animals and smaller than in the seal and porpoise. The pyramids are prominent thick bundles. The gracile and cuneate nuclei (proprioceptive body sensitivity) are also well developed in the otter.
2. Harbor Seal (Phoca vitulina) Pinnipeds spend much of their time on land. They have very good vision and their hearing is acute. The pineal organ is present. Both pairs of limbs are present although more or less modified, thereby indicating high somatic sensitivity in general. Apart from important differences, seals share many features with terrestrial carnivores, e.g., large telencephalic hemispheres with a well-developed corpus callosum. The hemispheres are covered with highly convoluted neocortex and nearly conceal the large cerebellum in dorsal aspect. The pons is flat but extended (Fig. 13). The olfactory bulb and tract are small and thin, and the anterior commissure is small. Hippocampus and amygdala are large, and the habenula is well developed. The visual system is well developed (thick optic nerve, large and layered lateral geniculate body, very large superior colliculus). The myelencephalon is wide, with the auditory system generally being well developed (thick eighth nerve, large ventral cochlear nucleus, trapezoid body, lateral lemniscus, medial geniculate nucleus and inferior colliculus), but the tuberculum acusticum (dorsal cochlear nucleus) is very small as in whales (no pinna). The trigeminal system is very pronounced (vibrissae), with the trigeminal being much thicker than the vestibulocochlear nerve, whereas the facial nerve and nucleus are of normal size (Fig. 13). The inferior olive is larger than in land carnivores but very similar to theirs in structure.
Among the fiber systems, the cerebral peduncles are very large and the pyramids thick, whereas the gracile and cuneate fascicles are smaller than in terrestrial carnivores but thicker than in cetaceans.
D. Sirenians: Dugongs and Manatees
In comparison with plesiomorphic dolphins such as the susu and franciscana, sea cows have small brains (Fig. 14). At about the same brain mass (220 g), the body of the young dugong (Dugong dugon) is about 4.5 times as heavy as that of the franciscana. The relative size of the telencephalic hemisphere (with respect to the total brain) is similar to that in the dolphin. With respect to basal insectivores, however, the size index of the cortex in the young susu is 3.5 times higher than in the dugong. In sirenians, gyrification of the cortex is only rudimentary. Also, there is little underlying white matter surrounding the very large ventricles, and die size of the corpus callosum is moderate. In the frontal region, cortical thickness (gray matter) is averaged at an exceptional 4.0 mm, and histological analysis suggests a potential motor cortical field at the rostroventral tip of the hemisphere as well as extended potential somatosensory fields on the adjacent lateral (convex) surface. Presumably, the primary auditory field and visual fields are located on the posterior lateral surface of the hemisphere and in the dorsal occipital region as in many other mammals. The cell number in the neocortex is reported to be sparse, but the subdivision into cortical fields and the lamination are more distinct than in cetaceans. The molecular layer (I) is of normal width. Layer II, which is most conspicuous, contains densely packed small pyramidal neurons with long apical dendrites. In the potential visual cortex, layer IV is composed of small almost granular pyramidal cells. Layer V is most pronounced in the potential motor field, and layer VI contains unique neuron clusters (Rindenkerne) in the potential somatosensory, visual, and auditory cortices. Sea cows have small olfactory bulbs and thin, ribbon-shaped olfactory tracts with small olfactory tubercles (Figs. 14a and 14b). Nevertheless, the olfactory system as a whole is distinctly larger in the sea cow than in the dolphin, but the vomeronasal organ is also lacking. The anterior commissure is well developed. The hippocampus and fornix are reported to be even smaller than in plesiomorphic dolphins, and the mammillary bodies are small and not visible superficially. The visual system seems to be moderately developed (relatively thin optic nerve, nuclei of ocular muscle nerves highly reduced in size, small superior colliculus). A pineal gland is lacking. The brain stem of the dugong is small in comparison with that of the dolphin: The diencephalon is moderately developed (small visual thalamic nuclei) and the size increase of the mesencephalon with respect to body mass is very low. The myelencephalon is also rather small, probably due to the moderate size of the auditory and motor systems, and despite the large trigeminal nuclei and very thick nerve supplying the rostral part of die head (particularly the extremely important vibrissae). The long ascending and descending fiber systems are moderately developed. Whereas most components of the auditory system, such as the cochlear nerve, ventral cochlear nucleus, medial geniculate nucleus, and inferior colliculus, are more or less well developed (inferior colliculus distinctly larger than superior colliculus), the dorsal cochlear nucleus is very small. The facial nerve and nucleus are much better developed (motor activity and control of the muzzle in seaweed grazing). With reference to basal insectivores and in comparison with dolphins, the cerebellum is rather small in the sea cow. This may be partly attributable to the sluggish swimming behavior of this animal, which can hardly evade attacks by humans. The vermis, however, is relatively large as in aquatic carnivores (otter, seal). In the sea cow, the paraflocculus is also relatively large but much smaller than in cetaceans. Whereas the pyramids are thin.
Figure 13 Harbor seal brain in basal aspect, py, pyramidal tract. Scale: 1 cm.
The decussation in the cervical spinal cord is distinct, but the pyramidal tract cannot be followed further caudalward. The gracile and cuneate nuclei (proprioceptive body sensitivity) are fairly well developed. The inferior olivary complex in sirenians is characterized as being small, but it bulges at the basal surface of the myelencephalon, with its medial (accessory) and lateral (principal) nuclei being of similar size. As a whole, the inferior olive very much resembles that of ungulates, but is distinct from that of the elephant.
E. Cetaceans
Cetaceans presumably originated from an extinct basal ungulate group (Condylarthra, Mesonychidae) that also gave rise to the living artiodactyls and close relationships with single extant ungulate groups have been discussed as well. Although generally very sparse in the fossil record, skeletal parts can give a reasonable impression of the evolutionary changes from terrestrial ancestors to living cetaceans. Unfortunately, this does not hold true for the brain, which only is known from endocasts. The latter formed in the cranial vaults of fossilizing precetacean or cetacean mammals and can provide only limited information about the external morphology of the actual brain of those animals. Furthermore, the understanding of cetacean brains has been hampered in yet another respect: Comparative considerations led to the idea that, similar to primates, cetaceans could be derived phylogenetically directly from insectivores, which represent some kind of “ancestral group” for many extant mammals. Nevertheless, although the cetacean neocortex exhibits some features that seem to re-call the situation in insectivorous brains, a more promising approach would be to compare cetaceans with plesiomorphie paenungulates such as the hyracoids (hyraxes or conies), pro-boscids (elephants), generalized artiodactyls such as the trag-ulids (Fig. 1), and also so-called “advanced” (derived) hoofed animals. Such a reconstruction of the hypothetical ancestors’ brains on the basis of “closely” related extant mammalian groups would be of considerable value for understanding cetacean evolution in general.
Figure 14 Sea cow (dugong) brain: (a) mediosagittal aspect and (b) basal aspect. After Dexler (1913), modified. Asterisk, dorsal evagination of diencephalic roof; circle, choroid plexus and interventricular foramen; FC, facial tubercle; Ip, interpe-duncular nucleus; L, lingula; OB, olfactory bulb; TG, trigeminal ganglion; 5.2, maxillary branch of trigeminal nerve. Scale: 1 cm.
In the franciscana (Pontoporia blainvillei), one of the least specialized living toothed whales (brain mass of the adult: 220 g; body mass: 35 kg), the encephalization index (EI: 5.7) ranges above the level of prosimians (lemurs, Tarsitts) and in the lower echelon of simians (monkeys, apes, human), whereas the EI level of marine dolphins (13.5) is above that of simian primates but clearly below that of humans. From what we know today, the franciscana brain shows all the features typical of toothed whales and mostly of whales, in general: (1) a large telencephalon and, at the same time, total loss of the rostral olfactory and the whole accessory olfactory system, and the reduction of the remaining paleocortex. The archicortex and some other components of the limbic system are verv small, but the limbic cortex seems to be well developed. The neocortex is veiy much maximally extended and convoluted. As in primates, the neocortex is by far the largest brain structure in river dolphins and other cetaceans. Its size index is higher in river dolphins than the average of prosimians but clearly lower than that of simians. Even higher values for the neocortex can be expected for marine dolphins. Giant cetaceans are difficult to interpret because their brains, although approaching 10 kg in total mass, are dwarfed by their huge bodies. In addition to the neocortex, the striatum is one of the most progressive tel-encephalic structures and indicates a high functional capacity of the motor systems. The amygdala, another component of the basal ganglia and belonging to the limbic system, is considerable in size, although its corticomedial component largely consists of paleocortex. (2) The thalamus is large due to the exceptional volume of different nuclei (medial, dorsal, ventral, medial geniculate, and pulvinar). (3) The large volume of the midbrain is significant and is due to the extreme size of some components of the auditory system. Thus, for example, the inferior colliculus is much larger than the superior colliculus. (4) Another major character is the large size of the cerebellum and particularly of the paraflocculus and posterior interposed nucleus as well as associated structures (elliptic nucleus, pons, inferior olive and accessory fiber tracts). (5) Finally, the medulla oblongata is, comparatively speaking, also veiy large due to the outstanding development of most of the auditory nuclei as well as those of the trigeminal and facial nerves.
These five adaptational trends seen in the dolphin brain are more or less paralleled in semiaquatie insectivores (Bauchot and Stephan, 1966) and other aquatic mammals (otter, pinnipeds) where growth of single brain regions surpasses the reduction in other regions such that total brain size increases. Sirenians, however, seem to be rather different in many respects. Nevertheless, in some ways and to some degree, these different groups of mammals have been regarded as “stages” of convergent adaptation to the aquatic habitat.
F. Comparative Aspects
According to the present state of our knowledge, aquatic mammals differ in the quantity and thickness of the neocortex and in the degree of gyrification of the telencephalic hemispheres. Whereas in pinnipeds and terrestrial carnivores the primary neocortical fields coincide in location, their position is rather different in dolphins in comparison with other mammals. The changes and differences in neocortical structure and composition found in aquatic mammals are not very well understood and cannot be explained to date functionally.
The general appearance of the neocortex in otters and pinnipeds corresponds fairly well to that of their terrestrial relatives. Sirenians show a small neocortex volume but a reasonable layering and cell density. In contrast, the neocortex in cetaceans is voluminous and extended but rather uniform, layer IV is weakly developed, and neuron density seems to be lowest within the Mammalia. Whether in sirenians the neuron clusters (Rindenkerne) in layer VI correlate with their vibrissae (bristles) and are equivalent to the “barrel fields” in rodents remains unsubstantiated to date.
The olfactory system of aquatic mammals seems to exhibit a clear relationship to their lifestyle. Whereas in aquatic carnivores the olfactory bulbs and tracts are clearly reduced in size, sea cows come even close to the baleen whales. Toothed whales only very rarely have vestigial olfactory tracts and are characterized by residues of a secondary olfactory cortex. Obviously, the olfactory part of the nose inherited from their terrestrial ancestors could not be adapted successfully to the requirements of aquatic life and therefore was reduced in extant aquatic mammals or even lost in cetaceans. As another example of such an adjustment, Jacobson’s organ (vomeronasal organ) is lacking in sirenians and cetaceans.
As in the cetaceans, parts of the limbic system (particularly the hippocampus) are reduced in some other aquatic mammals. Whereas in aquatic carnivores the hippocampus seems to be as large as in terrestrial mammals, sea cows and cetaceans have a minimal amount of archicortex. Other parts of the limbic system (e.g., fornix, mammillary body, mammillothalamic tract) are much reduced in toothed whales, but the anterior thalamic nuclei and the limbic lobe of Morgane are well developed. Here, we have a rather mysterious situation which, so far, defies explanation.
In the cetacean species investigated, the anterior commissure is generally very thin, obviously correlating with the reduction of its olfactory component. In sirenians and pinnipeds, the anterior commissure is neither as strongly reduced as in cetaceans (with respect to the whole brain) nor as thick as in the otter, and it is thin in comparison with that in land mammals. The corpus callosum is short and thin in dolphins but seems to be thicker in baleen whales. Its size (cross-sectional mediosagittal area) was shown to be inversely related to the mass of the brain (Tarpley and Ridgway, 1994). This potentially moderate interhemispheric connectivity may be related to the fact that neuronal density is exceptionally low in cetaceans and may indicate some interhemispheric independence as seen in the example of unihemispheral sleep in bottlenose dolphins. In sirenians, the corpus callosum is small in comparison to that in terrestrial mammals (ungulates) but it is well developed in aquatic carnivores (pinnipeds).
The basal ganglia of dolphins are well developed, particularly the nucleus basalis of Meynert, whose size seems to correlate positively with the neocortex (temporal lobe) and is maximally developed in cetaceans and primates. The caudate nucleus and putamen unite in a thick fundus striati, which encroaches on the surface of the basal forebrain in the area of the olfactory lobe (olfactory tubercle, diagonal band). In comparison with the porpoise, the putamen of the seal and otter is much smaller, but the pallidum is well developed. In the sea cow, the basal ganglia are small compared with those of other aquatic mammals.
The visual system shows a very different degree of development: Otters and seals have large eyes and central visual structures, whereas in cetaceans the eyes and optic nerves are of medium size and in sea cows small. It is not clear whether there is an inverse relationship in mammals between eyesight and the degree of adaptation to aquatic life.
The auditory system reaches its maximal development in the Cetacea. Whereas all aquatic and semiaquatic mammals exhibit the same bauplan, pinnipeds possess well developed but much smaller auditory structures, and in Sirenia most auditory components are developed only moderately. The dorsal cochlear nucleus, which is believed to be engaged in the integration of positional effects of the head and pinnae in the hearing process, is reported to be of very small size in aquatic mammals generally. This might correlate with the fact that in these animals the external ear is reduced in size or even lacking.
Most aquatic mammals are characterized by a well-developed cerebellum. The highest relative cerebellar mass is found in cetaceans (dolphins) and it is lower in pinnipeds and in the otter, whereas the good-natured and restful herbivorous sirenians have a comparatively small cerebellum. Within the cerebellum, there was obviously an allometric shift in the size relationship between the vermis and hemispheres during the adaptation to aquatic life, thus resulting in a small vermis but large cerebellar hemispheres in the fully adapted cetaceans. In aquatic mammals, the paraflocculus is very large or even the dominant part of the cerebellum. In the porpoise, the paraflocculus is connected to a specific cerebellar nucleus (posterior interposed nucleus), which may have evolved as an extension of the dentate nucleus and is very large. The paraflocculus is less developed in sirenians than in cetaceans, and even less so in the seal and otter. The latter approach the situation found in terrestrial carnivores, paenungulates, and ungulates, where the paraflocculus is already well developed, especially in the amphibious hippopotamus.
In cetaceans, the size of the pons and its nuclei seems to correlate with the size of the cerebellum and neocortex and with the massive projections to and from the huge elliptic nucleus as well as with the size of the rostral part of the medial accessory inferior olive.
The inferior olives of semiaquatic and aquatic mammals have been characterized by morphological criteria. As is the case for most components of the cetacean brain, experimental data are not available here. Cetaceans show an extreme thickening of their medial (accessory) olive, more accurately the rostral part of this subnucleus, which bulges at the base of the myelencephalon and seems to be a secondary acquisition. The caudal part of the cetacean medial accessory olive resembles in shape more that in sirenians (dugong) and in ungulates than that in terrestrial carnivores. In the harbor seal, where the medial accessory olive is the largest of the subnuclei, it is larger than its counterpart in terrestrial carnivores but by far smaller than that in cetaceans. A similar situation is found in the otter, in which the olivary complex, however, is somewhat smaller than in the seal.
The pyramids appear to be somewhat better developed in sirenians than in the harbor porpoise; their crossing is very distinct. In comparison, pinnipeds and the otter have thick pyramidal cords, possibly as a heritage of their terrestrial carnivore ancestors. In contrast, ungulates (artiodactyls and perisso-dactyls) tend to have moderately developed pyramids. With their very weak spinal pyramidal tract, cetaceans seem to show an “overlapping” of phylogenetic heritage and morphological as well as physiological adaptations to aquatic life, i.e., a reduction of the peripheral locomotor apparatus (hindlimbs and pelvic girdle) and a strong modification of the anterior limb and girdle (flipper, shoulder girdle).
VI. Strategies in Aquatic Adaptation
In the semiaquatic and most aquatic mammals investigated (insectivores, otters, pinnipeds, river dolphins, marine dolphins), the brain taken as a whole and a number of its components show a convergent moderate to strong increase in size with respect to potential terrestrial relatives within their groups. There is considerable variability, however, in the different brain structures of the species concerned. Whereas in semiaquatic insectivores and the sirenians the total brain and telencephalon are moderately enlarged for the body in comparison with basal insectivores, the otters, pinnipeds, and particularly dolphins are increasingly more encephalized. Otters and pinnipeds have larger brains than their potential terrestrial relatives, but nothing is known about their quantitative composition.
In some groups of semiaquatic and aquatic mammals (single species of insectivores, otter, pinnipeds), the limbic system is well developed. In cetaceans and sirenians, however, the situation is more complicated. In cetaceans, the archicortex (hippocampus) is very small, as are the fornix and mammillary body, but not the amygdala. Moreover, the limbic lobe as a transitional area between archicortex and adjacent neocortical areas is large. It might be concluded, therefore, that the limbic system in cetaceans differs markedly from that in terrestrial mammals. In sirenians, the hippocampus was reported to be even smaller than in cetaceans and its size is obviously minimal within the Mammalia. It is unclear whether the strong reduction of the hippocampus, which in mammals is primarily concerned with attentiveness and vigilance, and serves as an intermediate store for all kinds of information and their “labeling” (short-term memory), correlates with the strong reduction (baleen whales) and even loss of the rostral part of the olfactory system (toothed whales). If we subscribe to the principle of a central position for the hippocampus in informational processing of mammals, we are unable to explain the coincidence in size between two groups otherwise so disparate in brain volume, physiology, and behavior, and so different from other mammals. The small size of the hippocampus, however, could explain why dolphins have difficulties learning new activities that are not already part of their normal lives. In this respect, the extremely large surface area and the considerable volume of the dolphin cortex, which deviates much in architecture from the pattern seen in “progressive” terrestrial mammals, indicates that dolphins may possess an “intelligence” very different from our own as the sum of their adaptations to the aquatic environment during approximately 50 million years of separate evolution.
In conclusion, it seems that, in most cases, the adaptation of mammals to aquatic life, including the necessity of three-dimensional locomotion and diving in a turbid or dark environment, causes (1) an increase in the size of the brain due to the growth of special functional systems such as the auditory and motor systems, (2) an increase in neocortex volume, and (3) the strong reduction or even loss of the rostral olfactory system and its possible compensation or replacement by the trigeminal system. Interestingly, toothed whales and sirenians as the most advanced groups in terms of aquatic adaptation differ in a number of major characteristics. With respect to the basal insectivores (Stephan), even plesiomorphic odontocetes are much more encephalized than sirenians, and most of their brain components and functional systems show a distinctly stronger growth (earlier discussion). The profound differences in the morphology and quantitative composition of the brain in dolphins and sirenians may be related to the contrasting behavior in these animals. Whereas dolphins are extremely agile, swift animals with an intense social life, hunting different types of highly mobile prey with a powerful sonar detection system, sirenians have no sonar and are sluggish plant eaters with a thick integument and limited diving capabilities.
Concerning neocortex morphology, cetaceans and sirenians differ with respect to its thickness, extension, arealization, degree of layering, and presumably neuronal density. Taken as a whole, it seems that in contrast to the situation in the sirenians the extended neocortex of dolphins is responsible for the processing of large amounts of acoustic data needed for orientation, feeding, and social communication. Because of the extreme propagation velocity of sound in water, the time intervals left over for meaningful decisions are very short for swift swimmers and active hunters. A large number of modules in the extended cetacean neocortex may facilitate the quick scanning and subsequent reconstruction of ephemeral two- or three-dimensional images of their “complicated” environment.
.