Clinical diagnosis
While axial back pain is common in scoliosis, Smith et al (2008) showed that neurological symptoms and deficits are also frequently found in these patients. The incidences of back pain and radiculopathy were found to be 99 percent and 85 percent, respectively. Neurogenic symptoms typically arise from the concave side of the curve from asymmetric disc collapse and resultant neural foraminal stenosis. In addition to axial pain and neurogenic symptoms, spinal imbalance can manifest in late presentation. With the patient standing up without hip or knee bending, coronal balance can be evaluated with a plumb line from the C7 spinous process. Normally this plumb line should intersect the gluteal cleft in a balanced spine. Gross sagittal balance can be determined by evaluating the relationship of the pinna of the ear to the greater trochanter of the femur.
Treatment
Non-operative treatment aims to control pain and function. This includes modalities such as medications, physical therapy, activity modification, orthotics, and injections. Numerous studies have shown that conservative treatment does not lead to improved pain and function compared with surgery (Bridwell et al, 2009; Smith et al, 2009; Glassman et al,2010). In a non-randomized, prospective study looking at 123 patients with a 2-year follow-up, Glassman et al (2010) questioned the cost-effectiveness of non-operative treatment when the average cost over 2 years was $10,815 US.
Thus, surgical management has been preferred. Like other spinal conditions, surgery is only entertained after conservative treatment has failed and the patient is presenting with refractory pain limiting function, progressive deformity or neurologic deficits. Smith et al (2009) have shown that surgery can lead to better outcomes in back pain, leg pain, disability, and health status after 2 years compared to non-operatively treated patients. This is in the face of greater pre-operative back pain, leg pain, and functional disability. Grubb et al (1994) also showed that pain relief was associated with a solid fusion, and surgically managed patients demonstrated improved standing and walking.
While the goal of adolescent idiopathic scoliosis is to prevent progression of deformity and subsequent sequelae such as pain and neurological symptoms, the objectives of surgical care for degenerative scoliosis are to improve current pain and neurologic symptoms, and to restore normal spinal balance, particularly in the sagittal plane; all while maintaining as many mobile segments as possible. Although surgery has been shown to be more effective than non-surgical treatment, there is no consensus regarding the optimal approach and the levels to be included. This is due to the variety of clinical presentations and extent of disease and lack of clear evidence-based literature on approaches. The number of levels to involve in a fusion has been debated. Cho et al (2008) demonstrated that short fusion is a viable method in patients with small Cobb angles and good global balance. In their study, the average Cobb angle in patients receiving short fusion (average was 3 levels) was 16 degrees. Careful assessment of the global alignment must be performed to prevent progression of deformity prior to performing instrumented fusion. The inclusion of L5-S1 is also debatable. While stopping the fusion at L5 reduces perioperative complications and chances of pseudoarthrosis, the theoretical advantages of including this segment include complete sagittal balance correction and obviating future revisions due to degenerative changes at the L5-S1 level (Bridwell et al, 2003). Some clear indications to extend fixation to the sacrum include spondylolisthesis, stenosis requiring decompression, and degenerative disc changes at the L5-S1 level.
Combined anterior and posterior approaches provide presumed circumferential fusion and generous sagittal correction. However, they are associated with high perioperative complication rates as shown in Berven et all’s retrospective study (2003). Despite achieving good sagittal correction, 32 percent of the patients developed perioperative complications including infections, dural tears, pneumonia, and acute renal failure. Overall, 40 percent needed repeat surgery for various causes including revision of fusion, hardware complications, and infection. With recent advances in instrumentations and techniques, circumferential fusion and deformity correction can be performed through a posterior-based approach. Interbody fusion can be done through the posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) techniques via a posterior-based approach. Crandall and Revella (2009) demonstrated equivalent results between these posterior-based interbody fusion approaches and anterior-based interbody fusion technique. Another recent technique to lessen the perioperative complication rates is the lateral lumbar interbody fusion through a minimally invasive trans-psoas approach (Figure 2). Although it has a steep learning curve, this method can provide lower complication rates, lower blood loss, and shorter hospital stay (Mundis et al, 2010).
In a recent retrospective study comparing surgical outcomes between decompression alone, decompression with limited fusion, and decompression with full curve fusion, Transfeldt et al (2010) showed that decompression alone had the lowest rate of complications followed by decompression and limited fusion, while the decompression and full curve fusion group had the highest rate of complications. On the other hand, post-surgical satisfaction questionnaire showed that the group with the full curve correction had the highest satisfaction rate, while the decompression alone group had the lowest satisfaction rate. Therefore, in spite of higher complication rates associated with full curve correction, patients subjectively prefer global curve balance through full curve correction.
Isthmic spondylolisthesis
Introduction
Spondylolisthesis is the slippage of one vertebral body on another. Isthmic spondylolisthesis is a common condition encountered in adolescents and adults and involves a defect of the pars interarticularis, which is the junction where the lamina and inferior facet meet with the pedicle and superior facet (Figure 3). This leads to a disconnect between the anterior and posterior elements of the vertebra, leading to slippage (olisthesis). The most common level is at the L5-S1 level (Figure 3), with the pars defect commonly discovered on L5 (Figure 4). The reason for this could be due to the fact that as one goes caudad on the lumbar spine, the pars get thinner.
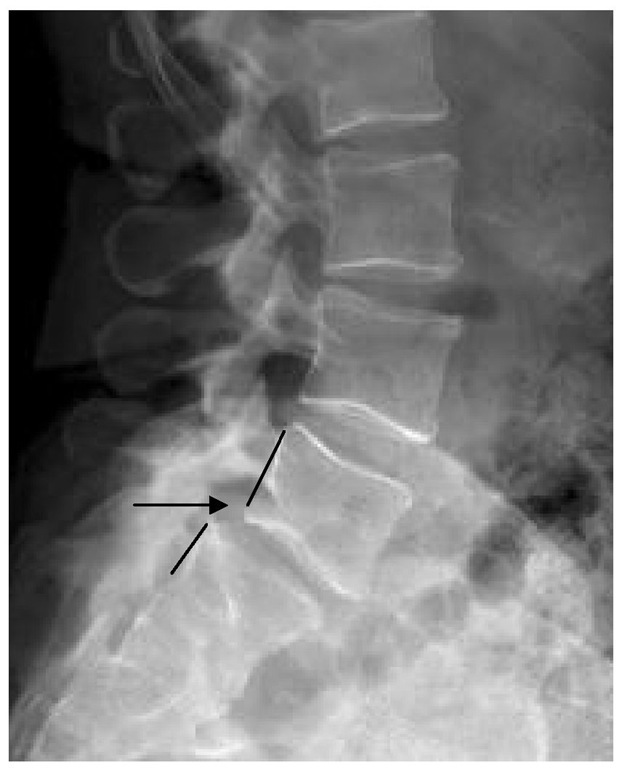
Fig. 3. Isthmic spondylolisthesis.
Lateral radiograph of the lumbar spine showing less than 25% spondylolisthesis at the L5-S1 level.. Notice the posterior cortices of L5 and S1 vertebral bodies do not line up.
This, coupled with the fact that the L5-S1 junction endures a lot of stresses, places this level at a high risk for isthmic spondylolisthesis. Contrary to degenerative spondylolisthesis (DS), the isthmic subtype is more common in males. The incidence of pars defect is estimated to be 4 to 6 percent in the general population (Meyerding, 1932; Boxall et al, 1979; Taillard, 1976). In their prospective study, Frederickson et al (1984) reported an incidence of 4.4 percent of pars defect and 2.6 percent of spondylolisthesis at the age of 6. At adulthood, the incidence of pars defect is 5.4 percent while spondylolisthesis is 4 percent.
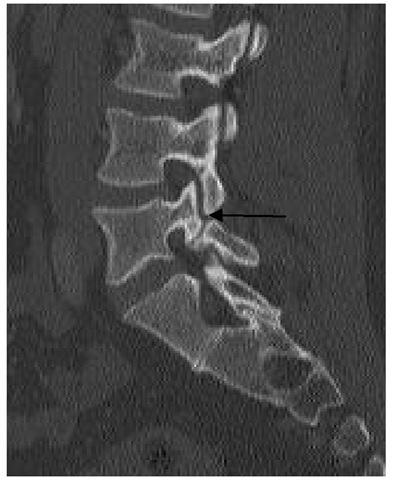
Fig. 4. Pars defect.
Sagittal reformat cut on computed tomography showing disruption of L5 pars interarticularis.
Natural history
In a 45-year follow up, Beutler el al (2003) showed that subjects with unilateral pars defects did not develop slippage. In those with bilateral pars defects without initial slippage, half showed no further slippage while the other half slipped a mean of 24 percent. Also, progression of the spondylolisthesis slowed with each decade and there was no association of slip progression and low back pain. Saraste (1987) showed that risk factors for low back symptoms were slippage greater than 25 percent, pars defect at the L4 level, and early disc degeneration.
Diagnostic imaging
Just like in DS, the lateral standing radiographs can depict spondylolisthesis and often the pars defect. If the pars defect cannot be seen on the lateral view, 30-degree oblique lateral views can be obtained. Computed tomography (CT) can provide the best bony details if still suspecting pars defect (Figure 4). Bone scan can aid in detecting stress fracture or reaction.
Clinical diagnosis
Most people with isthmic spondylolisthesis are asymptomatic. Back pain is generally worsened by activities and relieved with rest. This pain can be caused by lumbar hyperlordosis, which is associated with tight hamstrings. Occasionally, a step-off deformity of the spinous processes can be palpated adjacent to the level of the spondylolisthesis. Neurologic symptoms are usually in a radicular and dermatomal distribution due to impingement of the exiting nerve root, which is frequently L5 for the L5-S1 level. The site of impingement is at the site of the pars defect where the body forms hypertrophic fibrocartilaginous tissue or Gill lesion in an attempt to heal the defect.
Treatment
Most people with symptomatic isthmic spondylolisthesis improve with non-surgical treatment. This includes nonsteroidal anti-inflammatory drugs, activity modification (not including prolonged bedrest), and physical therapy. Radicular symptoms can be treated with epidural or transforaminal injections. Indications for surgery include failure of conservative therapy, progressive instability and/or neurological function, and intractable back or leg pain specific to the spondylolisthetic level. Surgical management of isthmic spondylolisthesis shows favorable outcomes compared to non-surgical treatment. In a prospective, randomized study comparing posterolateral fusion with an exercise program, Moller and Hedlund (2000) demonstrated that the surgical group had better functional outcome based on the Disability Rating Index and pain reduction.
The general basis of surgery for this condition is stabilization of the spondylolisthesis with or without decompression of affected neural structures. Since decompression alone fails to stabilize the spondylolisthesis, the options include decompression and non-instrumented posterior fusion, decompression and instrumented posterior fusion, decompression with posterior fusion augmented with anterior column support in the form of interbody fusion, and direct pars repair.
Controversy exists about non-instrumentated versus instrumentated posterior fusion. In a 5-year prospective randomized study comparing the two techniques, Bjarke et al (2002) showed that patients with non-instrumented posterior fusion had better clinical outcomes than their counterparts, and there was no difference in fusion rates between the two groups. Moller and Hedlund (2000) also echoed similar findings in that instrumentation does not add to the fusion rate nor improve clinical outcomes. Proponents of instrumentation claim that it can attain slip reduction and can restore sagittal alignment. Pertaining to reduction, Poussa et al (2006) showed that patients receiving in situ fusion had better outcome scores compared to the group that had reduction and fusion. Moreover, the reduction group had more neurologic complications and pseudoarthroses than the in situ fusion group. Hence, instrumentation and slip reduction have not been shown to have clear superiority over non-instrumentation and in situ fusion.
The addition of anterior support with interbody fusion theoretically provides circumferential fusion sites. Multiple studies have shown positive effects of anterior support with interbody fusion in high-grade spondylolisthesis (Helenius, 2006; Molinari, 1999, Shufflebarger, 2005). These include better functional outcomes and fusion rates. On the other hand, the use of interbody fusion is debatable for low-grade spondylolisthesis. Standalone interbody fusion without posterior instrumentation is discouraged in this condition due to high rates of failure such as cage migration (Button et al, 2005).
The theoretical advantage of direct repair of the pars defect relates to its ability to preserve motion compared with fusion, possibly leading to decreased degeneration in the adjacent segment. Although direct repair has been proven to be successful with low-grade spondylolisthesis in the short-term period (Morelos, 2004), it has not been shown to be as effective in the long-term period as initial improvement in functional outcomes declined with time and the adjacent segment degeneration phenomenon was comparable to those who received posterior fusion (Schlenzka et al, 2006). However, the method of direct repair shown in Schlenzka et al’s study involved cerclage wiring, whereas today’s fixation typically involves screws/hooks and/or rods (Figure 8). As a result, it is unknown whether today’s technology could prove otherwise and long term follow up studies are needed.
Degenerative spondylolisthesis
Introduction
Degenerative spondylolisthesis (DS) is a condition generally found in females older than 40 years of age. The usual level of involvement is L4-L5, with L4 slipping anterior to L5 (Figure 5). The cause of this is presumed to be a result of structural degenerative changes in disc and ligaments, more importantly the facet capsules. In a review of magnetic resonance imaging (MRI) in 140 subjects, Boden et al (1996) suggested that more sagitally oriented facets might be the cause of DS.
Natural history
Matsunaga et al (1990) studied the natural course of DS by observing 40 patients from 5 to 14 years. Slip progression was seen in 12 (30 percent) of the patients, but this did not correlate well with clinical symptoms. Meanwhile, 4 of the 28 patients who did not show progressive slip displayed clinical deterioration. Therefore, there is a lack of correlation between progressive slip and clinical symptoms. Also, the study infers that there is no correlation between degenerative changes, such as intervertebral disc narrowing, spur formation, subcartilaginous sclerosis, or ossification of ligaments, and slip progression, hence, suggesting that these anatomic changes may act to stabilize the spine.
Diagnostic imaging
Since DS is a dynamic condition involving instability of the spine, the preferred radiological imaging study is a lateral radiograph, in the standing position. Dynamic flexion and extension views can be added for further inspection of the instability. In a study by Boden and Wiesel (1990) looking at dynamic flexion and extension views, 90 percent of asymptomatic volunteers had 1 to 3mm of translation, therefore, it was considered that anything more than 4mm is abnormal. Slippage is graded based on the percentage of antero-posterior displacement on the vertebral body. Grade 1 equates to less than 25 percent of displacement on the caudad vertebral body; grade 2 is up to 50 percent; grade 3 is up to 75 percent; and grade 4 is up to 100 percent. Additionally, supine views are not helpful since this position may reduce the slippage. Although MRI portrays a static condition, a study by Chaput et al (2007) showed that large (>1.5mm) facet effusions are highly predictive of DS at L4-L5.
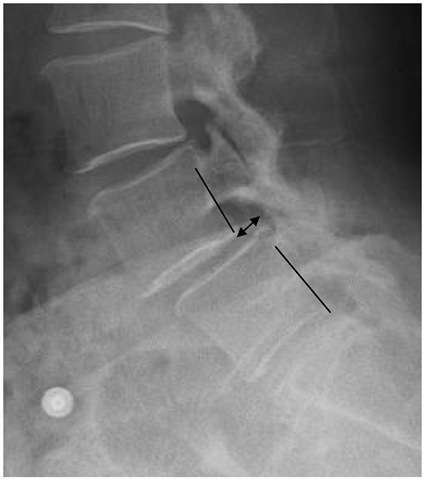
Fig. 5. Degenerative spondylolisthesis.
Lateral radiograph of the lumbosacral spine showing grade 1 spondylolisthesis at the L4-5 level. Notice the posterior cortices of L4 and L5 vertebral bodies do not line up. The percentage of displacement is approximately 20-25 percent of the vertebral body of L5.
Clinical diagnosis
Axial back pain in DS is frequently associated with back extension, whereas back pain in discogenic back pain is classically related to sitting and flexion. Other features of the condition can mimic spinal stenosis and lead to neurogenic claudication. The predominant symptom is pain, radiating from the buttock to the legs, and commonly involves bilateral legs. The neurogenic symptoms do not resemble radicular symptoms in affecting a specific dermatome, but may be diffuse in nature. If there are associated radicular signs, L5 is the most commonly involved root. Also, neurogenic claudication must be differentiated with vascular claudication when diagnosing DS.
Treatment
Generally, a comprehensive course of non-surgical treatment is the first line unless the patient exhibits any sign of neurological deterioration. This is defended by Matsunaga et al’s (2000) study showing that 76 percent of his sample size remained without neurological deficit at the 10 year follow up. Those who have failed conservative treatment and display increased or persistent pain, with or without neurologic symptoms, may be considered for surgery. The Spine Patient Outcomes Research Trial (SPORT) depicts the benefits of surgical treatment in patients with DS associated spinal stenosis. They followed 607 subjects for 4 years and rated their progress with outcome measures including, SF-36 and ODI. Despite their high cross over rate between surgical and non-surgical treatment groups, their conclusion was that patients with DS treated with surgery showed better improvement in pain and function during the 4 year follow up. Another shortcoming of this study was that it did not compare different types of surgical techniques. However, there are numerous studies that offer insights into the optimal surgical treatment.
The surgical options include decompression alone, decompression with posterior non-instrumented fusion, decompression with posterior instrumented fusion, and decompression with posterior fusion and anterior column support. Several papers have clearly shown that posterior non-instrumented fusion in conjunction with decompression leads to better clinical outcome than decompression alone in DS patients (Herkowitz, 1991; Mardjetko, 1994). As far as whether or not to add instrumentation to the fusion is still debatable. Fischgrund et al (1997) demonstrated in a prospective, randomized study comparing instrumented fusion with non-instrumented fusion, that fusion rate at 2 years was better in the instrumented group compared to the non-instrumented group. In spite of this, clinical outcome was similar for both groups. As a result, it is up to the physician’s discretion to determine when it is appropriate to place instrumentation in this setting of spinal instability. Similarly, there is no convincing data to support the routine use of anterior column support, such as interbody fusion, in addition to posterior fusion. The purported advantages of this would be restoration of disc height and neuroforaminal space, circumferential fusion leading to higher likelihood to fuse, and better sagittal alignment restoration.