Tangier disease is a rare recessive disorder in which patients have almost no HDL. Cholesterol ester accumulates in macrophages and macrophage-rich tissues like spleen and liver. Familial hypoalphalipoproteinemia (FHA) is a very common dominant disorder in which people have low HDL (typically <30 mg/dl) and suffer from premature heart disease even without an elevation in LDL.
Approximately 40% of patients with premature coronary heart disease have low HDL, making it the most common lipid disorder of heart disease patients.
Tangier disease and FHA are caused by mutations in the same gene, ABCA1. Tangier disease patients are ho-mozygous (or inherit two different mutant alleles). FHA patients are heterozygous for mutations at the ABCA1 locus. ABCA1 mutations lower HDL because they prevent phospholipid and/or cholesterol from effluxing and becoming associated with apo-A1 and apo-A2. In the absence of sufficient lipid, apo-A1 is rapidly cleared by the kidneys.
TABLE VI Scavenger Receptor Family
|
Name |
Ligands |
Tissue locations |
|
Class A |
||
|
SR-AI, SR-AII |
Acetylated LDL, oxidized LDL, polyanions, crocidolite asbestos, bacterial endotoxin, lipoteichoic acid |
Macrophages, (Kupffer cells, histiocytes, microglial cells), some endothelial cells (low level) |
|
MARCO (SR-AIII) |
Bacteria |
Macrophages |
|
Class B |
||
|
SR-BI |
HDL, LDL, modified lipoproteins, anionic phospholipids, acetyl LDL |
Highest expression in steroidogenic cells (adrenal, ovary, testis) and hepatocytes, lower level expression seen in absorptive epithelial cells of the proximal small intestine, lactating mammary gland, low levels observed in all cultured cells |
|
CD36 |
HDL, LDL, modified lipoproteins, anionic phospholipids, fatty acids, collagen, malaria-infected erythrocytes |
Adipose, macrophages, epithelial cells, monocytes, certain endothelial cells, platelets |
|
Croquemort |
Apoptotic cells |
Drosophila macrophages |
|
Class C |
||
|
SR-CI |
Multiple polyanions, including modified LDLs, poly(I) |
Drosophila macrophages |
|
Class D |
||
|
Microsialin (CD68) |
Modified lipoproteins |
Macrophages, Kupffer cells, endothelial cells |
|
Class E |
||
|
LOX-1 (SR-E1) |
Oxidized LDL |
Endothelial cells |
|
Class F |
||
|
SREC |
Oxidized LDL |
Endothelial cells |
|
FcgR2-B2 |
Oxidized LDL |
Macrophages |
Why do mutations in ABCA1 lead to premature heart disease? ABCA1 fulfills a rate-limiting step in the pathway by which cells get rid of cholesterol, and thus might be a critical protector against cholesterol overload. With the exception of hepatocytes, cells are unable to catabolize large quantities of cholesterol and must therefore protect themselves from cholesterol overload by expelling cholesterol to an appropriate extracellular carrier. It is interesting that ABCA1 is especially abundant in macrophages; macrophages can become engorged with cholesterol esters and form foam cells in the arterial wall. A mutation in ABCA1 might therefore predispose an individual to atherosclerosis by impeding cholesterol efflux.
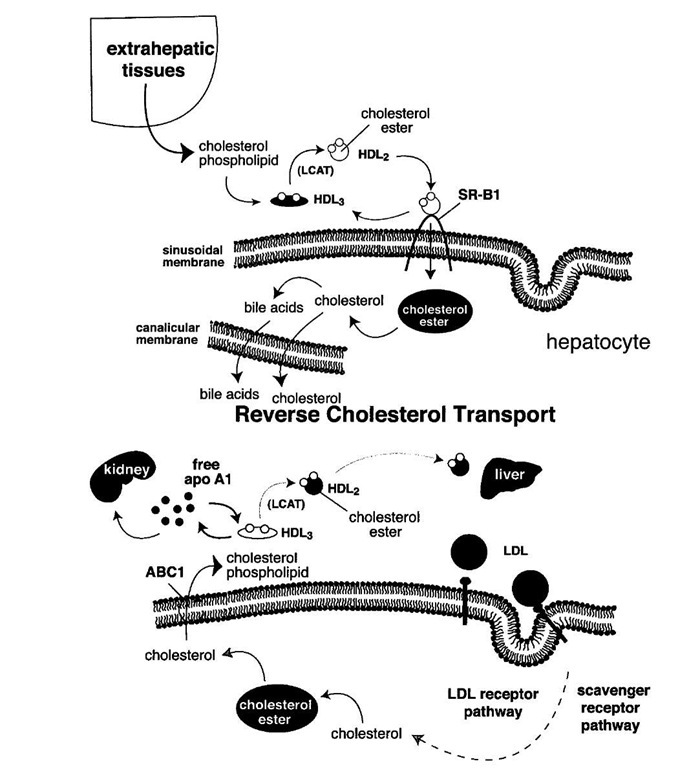
Do HDL levels correlate with the rate of reverse cholesterol transport? Studies of the expression of the SR-B1 receptor provide an answer to this question. When HDL binds to SR-B1 at the plasma membrane of the hepatocyte, it unloads its cholesterol ester cargo. The lipid-depleted apo-A1 then is cleared from the circulation, in part by the kidney. High levels of SR-B1 therefore lead to low HDL levels. However, under these circumstances, the transport of cholesterol to the liver is enhanced. When the diet is shifted from saturated to polyunsaturated fat, HDL levels typically drop. This drop is attributable to a rise in SR-B1 expression in the liver. In short, a decrease in HDL can be caused by imparied reverse cholesterol transport (extra-hepatic; Tangier disease) or by increased reverse cholesterol transport (liver; polyunsaturated fat).
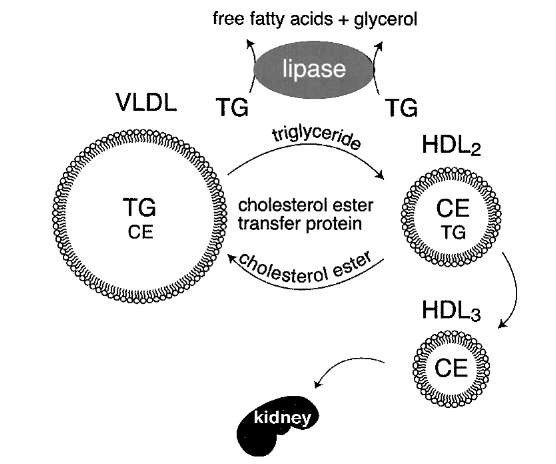
HDL metabolism is intimately connected with triglyc-eride metabolism. Clinicians know this because patients with hypertriglyceridemia almost invariably have low HDL levels. The cholesterol ester transfer protein (CETP)-catalyzed transfer of cholesterol ester from HDL to VLDL occurs with reciprocal transfer of triglyceride to HDL (Fig. 13). Lipoprotein lipase and hepatic lipase (another cell-surface-bound lipase) catalyze the hydrolysis of HDL triglyceride, reducing the size of the HDL particles. Hepatic lipase can also hydrolyze HDL phospho-lipids. Smaller HDL particles (HDL3) are removed from the circulation more quickly than larger HDL (HDL2).
FIGURE 12 The reverse cholesterol transport hypothesis. Extraheptatic cells accumulate cholesterol through the uptake of LDL and modified forms of LDL through the LDL receptor, other members of the LDL receptor family, and/or scavenger receptors. These cells efflux cholesterol and phospholipids to the extracellular milieu through a process facilitated by a membrane transporter, ABC1. Free apo-A1 interacts with the phospholipid and cholesterol to form HDL3 particles. LCAT esterifies the cholesterol to form discoidal HDL2 particles which then interact with the SR-B1 receptor at the surface of hepatocytes. Through that interaction, cholesterol esters are selectively taken up into the hepatocytes and hydrolyzed. The free cholesterol is then secreted into the bile or converted to bile acids. Much of this cholesterol is then excreted in the feces.
FIGURE 13 Inverse relationship between plasma triglyceride and HDL cholesterol levels. A higher level of VLDL correlates with lower HDL levels. Two processes simultaneously remove triglycerides from VLDL particles. First, lipoprotein lipase hydrolyzes the triglycerides to free fatty acids and glycerol. Second, cholesterol ester transfer protein (CETP) in the bloodstream catalyzes the exchange of triglyceride and cholesterol ester between VLDL and HDL, respectively. As HDL accumulates triglyceride, it is a substrate for lipoprotein lipase and hepatic lipase. This shrinks the HDL particles, causing them to be cleared by the kidneys.
The abundance of VLDL relative to HDL significantly influences HDL metabolism, probably due to enhanced exchange of triglyceride into the HDL particles. Individuals with genetically reduced levels of cholesterol ester transfer protein have extremely high HDL levels. Heavy exercise is also associated with increased HDL levels. Exercise increases the expression of muscle lipoprotein lipase. The resulting increase in VLDL lipolysis decreases the amount of triglyceride that can participate in the CETP lipid exchange process. This results in an elevation in HDL.