Chylomicrons and VLDL carry many apolipoproteins, among them apo-B and apo-E. Apo-B is a large (MW = 516 kDa) protein stably associated with the lipoprotein particle. The intestine produces a truncated form of the apoB, termed apo-B48, which is missing the carboxy-terminal half of apoB. The mechanism for the truncation involves a unique RNA editing event in the intestine that changes a Gln codon (CAA) to a STOP codon (UAA). Since only VLDL is converted to LDL, this means all apo-B in LDL is apo-B100 (Fig. 7).
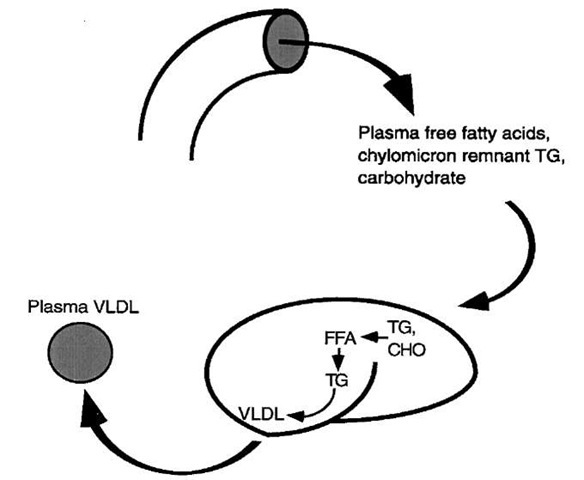
FIGURE 5 Sources of fatty acid for hepatic triglyceride synthesis. (1) Adipose tissue lipolysis, catalyzed by hormone-sensitive lipase, provides fatty acids that travel through the bloodstream to the liver. (2) Chylomicron remnants still carry some triglyceride and are cleared from the circulation by the liver. (3) Carbohydrate is converted to fatty acids when glycogen stores are maintained.
FIGURE 7 Chylomicrons contain a different form of apoB than VLDL or LDL. In the intestine, an RNA editing event introduces a stop codon in apo-B, resulting in a truncated protein product, apo-B48. VLDL is secreted with full-length apoB, apo-B100, and thus gives rise to LDL particles with apo-B100. The receptor-binding domain of apoB is at the C-terminal half of the protein, thus apo-B48 cannot bind to the LDL receptor; chylomicrons depend upon apo-E for receptor binding. Note: the particles are not drawn to scale; chylomicrons are about five times larger than LDL particles.
Main Features of VLDL and IDL Metabolism
• The liver is an important lipogenic tissue and exports newly synthesized triglyceride in VLDL particles.
• VLDL is synthesized in the liver and secreted directly into the bloodstream.
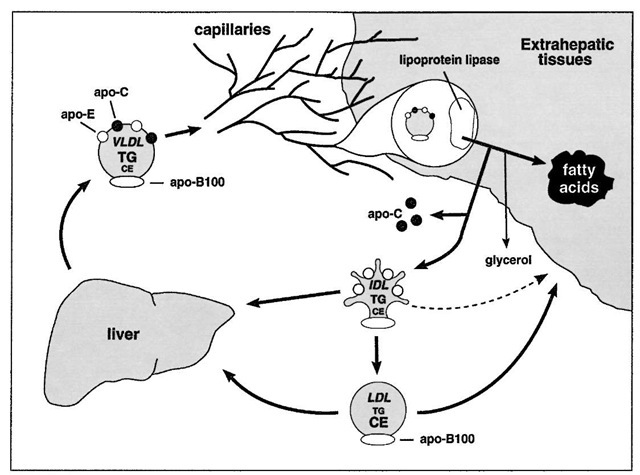
• As occurs with chylomicrons, VLDL is acted upon by lipoprotein lipase, on the surface of adipose and muscle capillaries. Unlike chylomicrons, VLDL remnants (IDL) are both cleared from the circulation and converted to LDL. This branch point is a factor that determines the rate of LDL production.
Whether or not a particle carries apo-B48 or apo-B100 has physiological importance. The carboxy-terminal half of apo-B is required for receptor recognition. Thus, apo-B100 can bind to cellular receptors (described below); apo-B48 cannot. For this reason, chylomicron remnants cannot be cleared from the circulation via apo-B48. Instead, chylomicron remnant clearance is mediated by another receptor ligand, apo-E. Indeed, genetic deficiency of apo-E leads to massive accumulation of cholesterol ester-rich chylomicron remnants and IDL in the bloodstream.
If chylomicrons and VLDL contain apo-E, why are they not cleared before they are acted upon by lipoprotein li-pase? One explanation is that the apo-E on chylomicrons and VLDL is masked by another protein, apoC-III. During lipolysis, the apoC-III protein falls off the particle, thereby exposing apo-E and permitting it to mediate particle clearance.
FIGURE 6 The VLDL ^ IDL ^ LDL pathway. VLDL is secreted by the liver directly into the bloodstream. Like chylomicrons, VLDL triglyceride is a substrate for lipoprotein lipase and is hydrolyzed while at the luminal surface of adipose tissue and muscle capillaries. The VLDL remnant (IDL), unlike the chylomicron remnant, can give rise to LDL. Some IDL is also directly cleared by the liver. LDL is cleared by the liver and by extrahepatic tissues.
• In terms of mass movement, export of triglyceride (TG) from the liver and intestine is where the action is. This means net movement to extrahepatic cells.
• Most of the plasma TG (in the fasting state, when no chylomicrons are present) is carried in VLDL. Thus, if fasting plasma TG is high, VLDL is high.
• Dietary fat is packaged into chylomicrons in the intestine. Excess endogenous substrate is converted into TG for export by the liver.
• Chylomicrons and VLDL are depleted of TG in the circulation, thus accomplishing their mission of delivering TG to peripheral cells.
• Chylomicron remnants are not converted to LDL; they are cleared by the liver.
• Only VLDL can be converted to LDL.
• In humans, most of the plasma cholesterol is carried in LDL. If plasma cholesterol is elevated without elevation of plasma TG, then LDL is high.