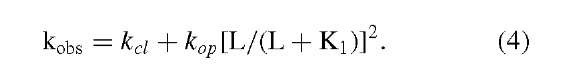Here, the emphasis is on kinetic techniques used to obtain the information needed to understand the mechanism of protein-mediated reactions that allow the transport of inorganic ions across biological membranes. A combination of structural, thermodynamic, and kinetic information is required to achieve this understanding. The use of X-ray crystallography, NMR measurements, and electron microscopy, to obtain structural information about proteins is described in detail elsewhere in this topic.
In 1976, Neher and Sakmann developed the single-channel current-recording technique. It is simple, convenient, and widely used for measuring the properties of a single, open receptor channel, such as its conductance, lifetime, and ion specificity. In brief, a glass pipet with an internal diameter of 1 to 2 / is attached to the surface of a cell membrane (Fig. 3A, left). Gentle suction is applied to isolate a small membrane patch within the glass pipet from the rest of the membrane (Fig. 3A, right). A silver chloride wire running from near the tip of the glass pipet to electrical recording equipment allows one to record the current flowing through single receptor-formed channels within the membrane patch (Fig. 3B). In the illustration, the deviation of the current from thebaseline represents the current flowing through a single nicotinic acetylcholine receptor-channel in the presence of 20 /M acetylcholine, which activates this channel. The Gaussian distribution of the current amplitude gave a peak centered at 3 pA. The exponential distribution of the time the channel remains open (the lifetime distribution) gave a value of 2.4 msec for the mean lifetime of the open channel, rop. This lifetime is a measure of the rate constant for channel closing ![]() (Fig. 2). Additionally, the technique allows one to determine conveniently the ion specificity of the channel from measurements of the current amplitude.
(Fig. 2). Additionally, the technique allows one to determine conveniently the ion specificity of the channel from measurements of the current amplitude.
In the case of transmembrane channels that open upon binding a specific ligand, how does one determine the other parameters of the channel-opening reactions? These are the dissociation constant of the neurotransmitter from the site controlling channel opening, the rate constant for channel opening kop and, therefore, the equilibrium constant for channel opening,![]() In principle, many of these constants can be determined from the lifetime of the closed state, the time interval between the openings of single receptor-channels (Fig. 3B). From the mechanism in Fig. 2, a plot of the number of closures observed within a definite time interval versus the time the channel was closed is expected to give a three-exponential distribution. From this distribution, the three different lifetimes, reflecting the constants to be determined, can be calculated. This evaluation requires many measurements to be made, which take time, and it is restricted to measurements made at low concentrations of neurotransmitters. At higher concentrations of neurotransmitter, the receptor becomes inactive, desensitized (in the millisecond time region) (Fig. 2) and the signal to be measured disappears. Additionally, we now know that many receptors on the cell surface exist in two forms, which desensitize with different rates.
In principle, many of these constants can be determined from the lifetime of the closed state, the time interval between the openings of single receptor-channels (Fig. 3B). From the mechanism in Fig. 2, a plot of the number of closures observed within a definite time interval versus the time the channel was closed is expected to give a three-exponential distribution. From this distribution, the three different lifetimes, reflecting the constants to be determined, can be calculated. This evaluation requires many measurements to be made, which take time, and it is restricted to measurements made at low concentrations of neurotransmitters. At higher concentrations of neurotransmitter, the receptor becomes inactive, desensitized (in the millisecond time region) (Fig. 2) and the signal to be measured disappears. Additionally, we now know that many receptors on the cell surface exist in two forms, which desensitize with different rates.
The desired, and missing, information that supplements results obtained with the single-channel current-recording technique can now be obtained by using a transient kinetic method with a microsecond time resolution, the laser-pulse photolysis (LaPP) technique. The usual rapid kinetic techniques that are suitable for investigating small molecules in solution had to be modified for use with membrane-bound proteins. The time resolution for equilibrating ligands in solution with membrane-bound proteins is less than might be expected. This is because a layer of water molecules (the diffusion layer) covers the membrane containing the proteins on the surface of relatively large objects like cells, or even membrane patches with diameters in the micrometer range. Ligands in the solution surrounding the membrane-bound receptors must diffuse through the diffusion layer and this process may become rate limiting. The steps needed to overcome this problem are illustrated in Fig. 4.
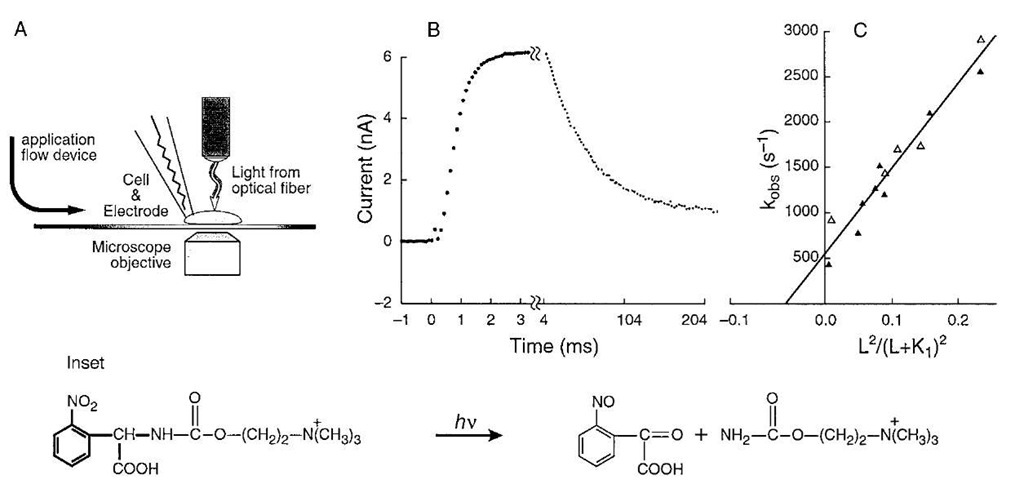
Photolabile precursors of neurotransmitters ("caged" neurotransmitters) that are biologically inactive have been developed. A photolabile precursor of carbamoylcholine, a stable analog of acetylcholine that activates the nico-tinic acetylcholine receptor, is shown in the inset to Fig. 4. Photolabile precursors of all the major neuro-transmitters are now available. This photolabile precursor of carbamoylcholine ([N-(a-carboxy-2-nitrobenzyl)-carbamoylcholine] is equilibrated with nicotine acetyl-choline receptors on the surface of a cell (Fig. 4A). At zero time, the compound is photolyzed, using a laser, by a single pulse within about 100 ^sec to give carbamoylcholine and a biologically inert side-product, a 2-nitroso-a-keto carboxylic acid (Fig. 4 reaction). An optical fiber carries the light beam to the cell, which is attached to a current-recording electrode (Fig. 4A). The technique for recording the current from all the receptors on the cell surface with high precision uses the same equipment as is used in the single-channel current-recording technique (Fig. 3). The increase in current that results when carbamoylcholine is liberated on the cell surface, due to the photolysis of caged carbamoylcholine is shown in Fig. 4B. The current is due the opening of receptor-channels on the cell surface and the flow of inorganic ions through them. In a different and slower time zone, the current then decreases due to receptor desensitization. In experiments with different neurotransmitter [glutamate, serotonin, y-aminobutyric acid (GABA), and glycine] receptors, conditions could be obtained in which the rise of the current follows a single exponential rate law. The observed rate constant for the rise time, kobs, is related to the rate constants for channel opening (kop) and closing (kci), the concentration of the ligand L that activates the transmembrane channel, and the dissociation constant of L, that is, Ki (Fig. 2):
The relationship between kobs and the concentration of neurotransmitter is given in Fig. 4C. The slope of the line gives the value of the rate constant for channel opening (kop) and the intercept on the ordinate gives the rate constant for channel closing (kcl).
This section outlined some approaches used to study the mechanism of proteins that transport inorganic ions across biological membranes. In the next section the properties of some individual proteins will be discussed.
FIGURE 3 The single-channel current-recording technique. (A) The tip of a borosilicate glass pipet, with a tip opening of 1-2 ^m is pressed against the membrane of a frog muscle cell (left). A slight negative pressure (20-30 cm H2O) is applied to the pipet for several seconds to form the seal between the membrane and the pipet (right). (Reproduced from O. Hamill et al. (1995). In "Single-Channel Recording," 2nd edition (B. Sakmann and E. Neher, eds.), p. 663, Plenum Press, New York.) (B) A typical current trace recorded using the single-channel technique, a rat myoball cell containing nicotinic acetylcholine receptors, and 20-^M acetylcholine (pH 7.2, 22°C, and Vm = -80 mV).
FIGURE 4 A BC3H1 cell (~20-/m diameter) containing nicotinic acetylcholine receptors, attached to an electrode for whole-cell current recording, is equilibrated with caged carbamoylcholine. A cell-flow device was used to equilibrate the cell surface with ligands in a solution flowing over the cell from the left. (A) A Candela SLL500 dye laser is used. Rhodamine 640 or sulforhodamine 640 laser dye, together with a second harmonic generator, produces wavelengths of 328 and 318 nm, respectively. The laser beam is introduced from an optical fiber of 200-/m diameter. The fiber is adjusted to be ~400 /m away from the cells so that the area illuminated around the cell has a diameter of 300 to 400 /m. The energy of the laser pulse emerging from the fiber is ~ 500/J and the pulse length is 600 nsec. By projecting visible light through the optical fiber, the cell is illuminated and the fiber properly positioned. Inset: Photolysis at 328 nm (using the Candela dye laser) of caged carbamoylcholine liberates a 2-nitroso-a-ketocarboxylic acid and carbamoylcholine, a stable and well-characterized analog of acetylcholine. The wavelength of 328 nm was chosen to avoid cell damage at lower wavelengths and too low a product yield at higher wavelengths. Current is recorded in the whole-cell configuration. (B) Whole-cell current induced by 200 /M released carbamoylcholine at pH 7.4, 22-23°C, and -60 mV. The points represent the digitized current data; the parameters of the heavy solid line were calculated from a first-order plot of the rise time and an observed first-order rate constant of 2140 sec-1. (C) Determination of kop, kcl, and K1 for the opening of nicotinic acetylcholine receptor channels in BC3H1 cells at pH 7.4, 23°C and -60 mV. The values of kobs determined from experiments shown in B are plotted according tc![]() The values of kop, kd, and K1 are 12,000 sec-1, 500 sec-1, and 210 /M,Respectively.
The values of kop, kd, and K1 are 12,000 sec-1, 500 sec-1, and 210 /M,Respectively.