The invention: A method for synthesizing amino acids by combining water, hydrogen, methane, and ammonia and exposing the mixture to an electric spark.
The people behind the invention:
Stanley Lloyd Miller (1930- ), an American professor of chemistry
Harold Clayton Urey (1893-1981), an American chemist who
won the 1934 Nobel Prize in Chemistry Aleksandr Ivanovich Oparin (1894-1980), a Russian biochemist John Burdon Sanderson Haldane (1892-1964), a British scientist
Prebiological Evolution
The origin of life on Earth has long been a tough problem for scientists to solve. While most scientists can envision the development of life through geologic time from simple single-cell bacteria to complex mammals by the processes of mutation and natural selection, they have found it difficult to develop a theory to define how organic materials were first formed and organized into life-forms. This stage in the development of life before biologic systems arose, which is called “chemical evolution,” occurred between 4.5 and 3.5 billion years ago. Although great advances in genetics and biochemistry have shown the intricate workings of the cell, relatively little light has been shed on the origins of this intricate machinery of the cell. Some experiments, however, have provided important data from which to build a scientific theory of the origin of life. The first of these experiments was the classic work of Stanley Lloyd Miller.
Miller worked with Harold Clayton Urey, a Nobel laureate, on the environments of the early earth. John Burdon Sanderson Haldane, a British biochemist, had suggested in 1929 that the earth’s early atmosphere was a reducing one—that it contained no free oxygen. In 1952, Urey published a seminal work in planetology, The Planets,in which he elaborated on Haldane’s suggestion, and he postulated that the earth had formed from a cold stellar dust cloud. Urey thought that the earth’s primordial atmosphere probably contained elements in the approximate relative abundances found in the solar system and the universe.
It had been discovered in 1929 that the Sun is approximately 87 percent hydrogen, and by 1935 it was known that hydrogen encompassed the vast majority (92.8 percent) of atoms in the universe. Urey reasoned that the earth’s early atmosphere contained mostly hydrogen, with the oxygen, nitrogen, and carbon atoms chemically bonded to hydrogen to form water, ammonia, and methane. Most important, free oxygen could not exist in the presence of such an abundance of hydrogen.
As early as the mid-1920′s, Aleksandr Ivanovich Oparin, a Russian biochemist, had argued that the organic compounds necessary for life had been built up on the early earth by chemical combinations in a reducing atmosphere. The energy from the Sun would have been sufficient to drive the reactions to produce life. Haldane later proposed that the organic compounds would accumulate in the oceans to produce a “dilute organic soup” and that life might have arisen by some unknown process from that mixture of organic compounds.
Primordial Soup in a Bottle
Miller combined the ideas of Oparin and Urey and designed a simple, but elegant, experiment. He decided to mix the gases presumed to exist in the early atmosphere (water vapor, hydrogen, ammonia, and methane) and expose them to an electrical spark to determine which, if any, organic compounds were formed. To do this, he constructed a relatively simple system, essentially consisting of two Pyrex flasks connected by tubing in a roughly circular pattern. The water and gases in the smaller flask were boiled and the resulting gas forced through the tubing into a larger flask that contained tungsten electrodes. As the gases passed the electrodes, an electrical spark was generated, and from this larger flask the gases and any other compounds were condensed. The gases were recycled through the system, whereas the organic compounds were trapped in the bottom of the system.
Miller was trying to simulate conditions that had prevailed on the early earth. During the one week of operation, Miller extracted and analyzed the residue of compounds at the bottom of the system. The results were truly astounding. He found that numerous organic compounds had, indeed, been formed in only that one week. As much as 15 percent of the carbon (originally in the gas methane) had been combined into organic compounds, and at least 5 percent of the carbon was incorporated into biochemically important compounds. The most important compounds produced were some of the twenty amino acids essential to life on Earth.
The formation of amino acids is significant because they are the building blocks of proteins. Proteins consist of a specific sequence of amino acids assembled into a well-defined pattern. Proteins are necessary for life for two reasons. First, they are important structural
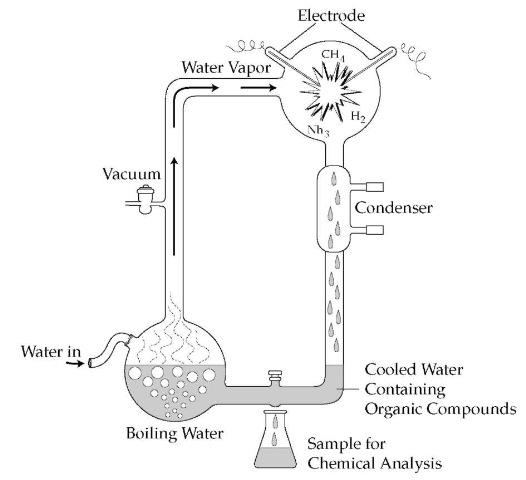
The Miller-Urey experiment.
materials used to build the cells of the body. Second, the enzymes that increase the rate of the multitude of biochemical reactions of life are also proteins. Miller not only had produced proteins in the laboratory but also had shown clearly that the precursors of proteins— the amino acids—were easily formed in a reducing environment with the appropriate energy.
Perhaps the most important aspect of the experiment was the ease with which the amino acids were formed. Of all the thousands of organic compounds that are known to chemists, amino acids were among those that were formed by this simple experiment. This strongly implied that one of the first steps in chemical evolution was not only possible but also highly probable. All that was necessary for the synthesis of amino acids were the common gases of the solar system, a reducing environment, and an appropriate energy source, all of which were present on early Earth.
Consequences
Miller opened an entirely new field of research with his pioneering experiments. His results showed that much about chemical evolution could be learned by experimentation in the laboratory. As a result, Miller and many others soon tried variations on his original experiment by altering the combination of gases, using other gases, and trying other types of energy sources. Almost all the essential amino acids have been produced in these laboratory experiments.
Miller’s work was based on the presumed composition of the primordial atmosphere of Earth. The composition of this atmosphere was calculated on the basis of the abundance of elements in the universe. If this reasoning is correct, then it is highly likely that there are many other bodies in the universe that have similar atmospheres and are near energy sources similar to the Sun. Moreover, Miller’s experiment strongly suggests that amino acids, and perhaps life as well, should have formed on other planets.
See also Artificial hormone; Artificial kidney; Synthetic DNA; Synthetic RNA.
