In the heyday of light-microscopy studies, intracellular bacteria were discovered in many organisms. In 1924 M. Hertig and S. B. 1 Wolbach observed bacteria in the cells of a mosquito (Culex pipi-ens). Hertig formally described them in 1936 as Wolbachia pipientis in honor of his collaborator in earlier work. Wolbachia was placed in the Rickettsiales. Its closest relatives are several pathogenetic bacteria (Cowdria and Ehrlichia) that are transmitted by arthropods to vertebrate hosts. After their discovery, little was reported on these bacteria until Yen and Barr in 1971 discovered that they were involved in causing the death of mosquito eggs when sperm of infected males fertilized the eggs of Wolbachia-free females. Since that time the interest in Wolbachia has increased rapidly, because Wolbachia is very common in arthropods (up to 70% of all insects) and causes a number of unusual manipulations of their host’s reproduction. These manipulations are interesting both from an academic point of view (evolution of sex, speciation, and conflict between different sets of genes within one organism) and from an applied point of view (can these bacteria be used to manipulate their host for our benefit?).
WOLBACHIA’S HOST MANIPULATIONS
Wolbachia is generally passed on only from an infected female to her offspring; infected males do not pass on their infection to their offspring. This can be understood because insect eggs are large and can harbor bacteria, whereas sperm are small and carry very little cytoplasm in which bacteria can be transported. Such asymmetry in the transmission ability of their host explains many of the effects that Wolbachia has.
Cytoplasmic Incompatibility
One of the best known and possibly the most common effect of Wolbachia is cytoplasmic incompatibility (CI). Several different forms of CI are known. In its simplest form, CI causes the death of embryos when a sperm from an infected male fertilizes the egg of an uninfected female. Consequently, in populations in which infected and uninfected individuals occur together, all possible crosses of infected females result in infected offspring. Uninfected offspring are produced only when an uninfected male mates with an uninfected female, whereas all the offspring of uninfected females that have mated with infected males die. This difference in offspring production causes the CI infection to rapidly spread through the population. In the fruit fly Drosophila simulans, the infection in California spread at a rate of approximately 100 km per year.
The underlying mechanism of CI is not yet understood, but it is believed that sperm of infected males are somehow modified during spermatogenesis so that only in eggs infected with the same type of Wolbachia can this sperm function properly or be “rescued.” This system of modification (mod) of the sperm and rescue (res) in the eggs is used to understand the possible CI types. For instance, if in one species two different Wolbachia infections exist, this could lead to two-way incompatibility because the modified sperm is not rescued by the eggs infected with the other type of Wolbachia. This leads to the death of all the embryos resulting from intertype crosses. Therefore, two infections within one species can cause genetic isolation of the two different subpopulations. This isolation may speed up speciation.
Not only is it possible that different populations within a species are infected with a different Wolbachia, but superinfections with several Wolbachia types are also common. Individuals infected with two different Wolbachia may be incompatible with individuals that are infected with only one of the two types. Sperm from doubly infected males may not be rescued in eggs that are singly infected. In these situations, the double infection should spread through the population, displacing the single infection.
The types of CI Wolbachia that have been discussed so far can all be classified as modify plus (mod+) and rescue plus (res + ). If we assume that both these functions are energy costly to maintain, one can imagine that once a complete population is infected with the mod+ , res+ variant, Wolbachia variants that do not modify the sperm any longer (mod— , res + . will spread relative to the modifying form (mod+ , res+ ). Finally, once all of the individuals carry the mod—, res+ variant, the population becomes vulnerable to invasion by mod—, res— variants. Mod — , res— host populations either may lose their infection or can be invaded by new mod+ , res+ variants. Examples of mod+ , res+ ; mod— , res+ ; and mod— , res— variants are all known. Mod+ , res— Wolbachia do not exist because the males would be incompatible with the females of their own population.
CI Wolbachia is thought to be promising in the spread of beneficial traits through a population. The idea is to genetically modify a Wolbachia with genes that inhibit the transmission of disease-causing organisms (malaria, dengue fever, etc.) through the insects that vector the disease. If this gene were inserted into a CI Wolbachia, the CI caused by the Wolbachia would lead to the spread of the bacterium through the vector population and all infected insects would be unable to transmit the disease. Wolbachia appears to be present in many different parts of the insect body. Consequently, the gene products inhibiting the disease organisms may also be delivered to various insect tissues.
Parthenogenesis-Inducing Wolbachia
Wolbachia inducing parthenogenesis in their hosts are known from many wasps and some thrips (Fig. 1) . Infected females can produce daughters both from fertilized and from unfertilized eggs. The cytogenetic mechanism through which Wolbachia cause unfertilized eggs to grow into females is known for a number of parasitoid wasps. Wolbachia exploits the normal sex determination in wasps. In this system, males arise from unfertilized eggs, which contain only one set of chromosomes, whereas females arise from fertilized
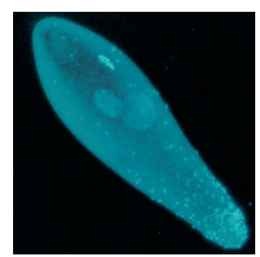
FIGURE 1 PI Wolbachia visible as light dots in a 4,6-diamidino-2-phenylindole-stained egg of the parasitoid wasp Trichogramma kaykai. The Wolbachia are concentrated around the area of the egg from which the future eggs of the wasp develop.
eggs, containing two sets of chromosomes. In unfertilized, infected eggs containing only a single set of chromosomes, Wolbachia causes a doubling in the number of chromosomes. This is accomplished through a modification of the first mitotic division in the egg, causing the egg to develop into a female.
In most populations in which parthenogenesis-inducing (PI) Wolbachia are known, the entire population consists of females. Feeding antibiotics to these females causes them to produce male offspring. In most instances, these males are not able to successfully inseminate the females of their own line. It is assumed that the populations have been parthenogenetic for such a long time that mutations have accumulated in the part of the genome involved with sexual reproduction. An exception to this situation is found in the tiny parasi-toid wasps of the genus Trichogramma in which, in many populations, both infected and uninfected individuals coexist. In these populations, males that are the offspring of antibiotic-treated mothers can successfully mate with infected females.
PI Wolbachia are of interest from the applied point of view as well. Many parasitoid wasps are reared on a large scale in insectaries for release in the field to control pest insects. Only female wasps are effective biological control agents because (1) they lay their eggs in or on the pest insect, (2) the wasp larvae will subsequently eat up the host insect, and (3) new wasps emerge from the remains of the pest insect. PI-Wolbachia-infected females are cheaper to produce than normal sexual wasps. No production effort is wasted on rearing of males that are worthless for biocontrol.
Feminizing Wolbachia
In many woodlice species (pill bugs: Crustacea, Isopoda), a Wolbachia causes infected genetic males to develop into functional females. The feminizing Wolbachia accomplishes this through the infection of the male-hormone-producing gland. Without this hormone, genetic males develop into females. The spread of such an infection through a population can result in a severe shortage of males. Because males are needed for reproduction, this has led to counteradaptations by the nuclear genes in such a way that males will be produced even if the individual is infected. The effects of the Wolbachia and the counteradaptations against the feminization have led to a dynamic system of sex determination in some species of woodlice.
Feminizing Wolbachia have also been discovered in a moth (Ostrinia scapularis: Crambidae). Infected individuals grow into females, but how the Wolbachia accomplishes this transition is not known.
Male-Killing Wolbachia
Many different microorganisms have evolved methods of killing only male offspring of infected mothers. Wolbachia have this ability in several lady beetles and butterflies. The advantage of killing male offspring to Wolbachia requires that the infected daughters somehow benefit from the death of their brothers. In the case of the lady beetles, this is most easily explained. Ladybird beetles generally lay their eggs in clusters, and the survival of the larvae is greatly enhanced if they find food soon after hatching. If an infected mother has laid a clutch of eggs, the daughters emerge and then consume the eggs containing their dead brothers as their first meal. Because males cannot pass on the Wolbachia, the enhanced survival of the infected females benefits the Wolbachia. The presence of the male-killing Wolbachia can lead to extreme sex ratios in the population, and just like in the case of the feminizing Wolbachia, counteradaptations are expected.
Male-killing Wolbachia occur with a high frequency in several species of the butterflies of the genus Acraea occurring in East Africa. This has led in some populations to extremely female-biased sex ratios and a concurrent change in behavior of the butterflies. Normally, males form leks where females would “choose” the male to mate with, but in these female-biased populations, females form leks where males “choose” their partners. Little is known about the mechanism that allows these Wolbachia to kill exclusively infected male eggs.
Other Phenotypes Induced by Wolbachia
Several additional effects are known to be caused by Wolbachia. In the parasitoid wasp Asobara tabida, three different Wolbachia are found; two of these can be removed without having much influence on the wasp, and the third one however is necessary for the wasp reproduction. If this strain is absent, the wasps become sterile. A strain of Wolbachia named popcorn causes both CI and a reduced life span in infected Drosophila melanogaster flies. This popcorn strain divides rapidly in the adult flies and results in the early death of the individuals.
In several cases Wolbachia have been found to cause different phenotypes depending on the host in which they are introduced. For instance, a Wolbachia causing CI in the moth Cadra cautella causes male killing in the moth Ephestia kuehniella. Similarly, a Wolbachia causing CI in Drosophila recens was introgressed into the closely related species Drosophila subquinaria, where it caused male killing in several lines.
PHYLOGENY OF WOLBACHIA
The phylogeny of Wolbachia has been studied extensively using several Wolbachia genes. Initially, the 16S ribosomal DNA sequence was used to derive the relationship between the different Wolbachia. From this work, it became clear that within the insect Wolbachia, two groups exist, later named A and B, that had diverged from each other approximately 60 mya. Later several other genes were used to get a finer scale picture of the relationship of the different Wolbachia to each other, and recently, a multi-locus sequence typing (MLST) system has been developed using five different Wolbachia genes to characterize strains. The MLST work has shown that frequent and extensive recombination takes place between different Wolbachia lines. This frequent recombination makes it likely that single gene phylogenies do not correlate with the phenotypes induced by the Wolbachia, unless the gene used for the phylogeny happens to be the one causing the phenotype. In addition, the publication of several Wolbachia genomes has made it clear that several mobile elements and phages are present. Not only do the Wolbachia genomes recombine with each other, but there are now several cases where parts of the Wolbachia genome have become integrated in the chromosomes of their hosts. Little is known if these integrated Wolbachia genomes remain functional or if they become completely silenced.
