The agility of insects certainly contributes to their reign as the most successful creatures in the animal kingdom. Few if any forms of terrain present an insurmountable barrier to all insects. By evolving variations on a basic body plan, they have achieved remarkable dexterity in a wide range of environmental niches. We find insects that walk slowly over floors, scurry under rocks, climb up walls and over ceilings, or jump over barriers that if scaled to human dimensions would represent achievements unattainable by the most accomplished athletes. These abilities have attracted the attention of engineers who study insect locomotion as inspiration for legged robotic devices.
Although these creatures are often described as “simple systems,” a close examination of their abilities reveals mechanisms that are elegant and not really simplistic. Their capabilities represent remarkable combinations of mechanical principles, neural control, and sensory input leading to efficient movement of leg joints. Although many aspects of these systems are economical in design and have been studied for many decades, they are only now beginning to be understood.
The problems inherent in insect walking and jumping encompass issues ranging from biomechanics to both central and peripheral neurobiological factors, as well as force development in muscle. To illustrate these points, we will examine the leg structures and neural circuits that produce walking and running in the cockroach. However, a variety of other insects have also been studied extensively by neu-robiologists and engineers, including stick insects, grasshoppers, and crickets.
To understand how an insect walks, we must subdivide the process. First, we must understand the movements of the legs and their constituent joints, and then we can begin to look at how muscles generate these movements and the circuits within the central nervous system that control motor activity. Over most walking speeds, sense organs of the limbs contribute to motor control by providing detailed information about the position, velocity, and the forces occurring in each leg. Therefore, we must also investigate the role of sensory input in the control of movement. Even with all this information, we will understand only how an animal can walk on a horizontal surface. But the aspects of walking in insects that are truly remarkable, and attract them to robotics engineers, are the abilities to run over and around obstacles, climb up walls, and jump over barriers. Often these adaptations occur through subtle changes in the basic pattern of locomotory behavior.
LEG MOVEMENTS
The walking movements of insects were accurately described in 1887 by Morgan, who in an era in which galloping of horses held the public attention wrote a letter to Nature entitled “The Beetle in Motion” that recounted the coordination seen in a tripod gait. In more detailed accounts in the mid-20th century, Hughes and Wilson noted that insects walk by moving their six legs in reproducible patterns. Each leg alternates between a stance phase, when the tarsus (foot) is on the ground and the animal is pushed forward, and a swing phase, when the tarsus is moved forward through the air. At slow speeds, the legs follow a metachronal pattern, moving from the hind legs to the middle and then to the front legs on either side. However, to move more rapidly, some legs must be moved at the same time; therefore, the insect shifts into a modification of the metachronal pattern called the tripod gait. Here the front and rear legs on one side of the animal move as a unit with the middle leg on the opposite side (Fig. 1A and 1B). This tripod alternates between swing and stance with the tripod made up of the remaining legs. The tripod gait is very stable, because at most speeds, the animal’s center of mass (CoM) remains within the base of support. However, at very high speeds, many insects make dynamic postural adjustments to stay upright. Remarkably, at exceptionally high speeds, the American cockroach Periplaneta americana has been seen to rise up on its hind legs and run with a bipedal gait. This gait is not statically stable (the cockroach would fall if it stopped), but represents a balance of forces that produces dynamic stability.
Each leg is made up of segments that are similar from leg to leg but differ in dimensions. From the most proximal to distal location, the leg segments are the coxa, trochanter, femur, and tibia, and a series of tarsal segments ending in a retractable claw (Fig. 1C). In the cockroach, the most important joints for walking are the coxa-trochanter (CTr) joint and the femur-tibia (FTi) joint. The CTr joint actually moves the femur relative to the coxa because the trochanter-femur (TrF) joint makes only small movements. Although flexion of the TrF joint can effectively rotate the tarsus, during many movements it acts mechanically as a fused joint. In other insects, relative proportions of these leg segments are changed to match the needs of specialized forms of locomotion. Thus, the hind leg of a locust has a very short coxa and a long muscular femur, making a powerful jumping leg.
Although the legs within a tripod move their feet as a unit, the joint movements and resulting forces are unique for each pair of legs. In cockroaches, the hind legs are specialized to propel the animal forward in walking. To accomplish this, the CTr joint and the FTi joint move in near synchrony (Fig. 1A) . This action allows these rotary joints to direct the movements of the tarsi (feet) in a line nearly parallel to the long axis of the animal’s body. The middle legs make similar movements, but with smaller joint (CTr) excursions. The orientation of this leg causes it to first brake and then accelerate forward movements of the animal.
The front legs make very different movements. Unlike the other two pairs of legs, the front legs make much greater use of the thorax-coxa (ThC) joint, which attaches the leg to the thorax. This joint has three degrees of freedom, similar to the ball-and-socket joint in a human shoulder. Movement of the ThC joint swings the front legs far forward much like an extending human arm. The CTr and FTi

FIGURE 1 Description of leg segments and movements in tripod gait. (A and B) Pictures from a high-speed video record of a cockroach walking on a lightly oiled plate. These images are taken at the beginning and end of one leg cycle. The legs forming one tripod (animal’s right front and rear legs and left middle leg) are indicated with triangles; the legs forming the other tripod are designated with circles. Lines connect the triangles to clearly indicate the tripod. Note that in (A) the left front and middle tarsi are very close together, almost overlapping. (C) Diagram showing the segments that make up a typical cockroach leg.
joints then move out of phase with each other as the foot is drawn back toward the body and then is pushed backward. The resulting ground reaction forces slow the forward movement of the body and keep the animal from losing control. Clearly, the neural control of this leg is much different from the control of the other two pairs of legs. Nevertheless, the sum of the ground reaction forces of all three pairs of legs is similar to that seen in the bipedal leg movements of a human.
Cockroaches can run extremely rapidly (up to 25 steps per second), ranking them as the fastest terrestrial animal (in steps per second). Running, which follows wind stimulation of the cerci (abdominal appendages) or tactile stimulation of antennae or body cuticle, is used to escape from predators. Although fast running shows a number of similarities to walking, it occurs so rapidly that it may actually be a separate behavior. Passive elastic structures in the legs can play an important role in generating such rapid locomotion, prompting cockroach running to be modeled as a mass on a spring.
MOTOR CONTROL OF LEG MUSCLE
The muscular anatomy of insect legs follows a proximal-to-distal arrangement that makes very good biomechanical sense. The largest and most powerful muscles are proximal or closer to the body. The muscles are smaller in more distal segments of the leg. With the most powerful muscles placed near the body, inertial effects in the distal part of the limb are reduced, allowing for more controlled movement. Also, the movements of the proximal joints act on the tarsus or foot through the relatively large lever arm of the intervening leg segments to the tarsus or foot. Thus, for example, relatively small movements at the ThC joint can greatly alter the orientation of the tarsal end point.
The most distal segment, the tarsus, is actually made up of a series of segments ending in a retractable claw that have again remarkable mobility. However, as with human fingers, the muscle for these segments is found in more proximal segments and imparts its movements via a long apodeme that serves the role of a tendon. Indeed, although the claw can be engaged by this muscle, there is no antagonistic muscle. Rather, the claw is disengaged and lifted from the substrate by a remarkably efficient elastic protein (resilin) found in the joints of the tarsal segments. The resilin acting like a spring on a screen door, is stretched when the claw is engaged, and causes the tarsus to be lifted automatically when the muscle is relaxed.
NEUROMUSCULAR SYSTEM
The neuromuscular arrangement of insects provides distinct advantages for motor analysis. Unlike vertebrate systems in which muscle cells fire action potentials, in insects most muscle fibers produce graded potentials when motor neurons fire action potentials. Variation in tension in a vertebrate muscle requires recruitment of more or fewer motor neurons. However, in arthropods, simply altering the frequency of action potentials in a single motor neuron can control tension. Furthermore, insect muscles are innervated by very few motor neurons. Often, especially in stance phase muscles (the muscles that extend the leg while the tarsus contacts the ground), there are only two motor neurons serving a range of muscles. These can be readily distinguished as either fast or slow motor neurons depending on the types of muscle contraction they produce. Slow motor neurons need a series of action potentials to generate significant movement, whereas fast motor neurons generate a reasonable twitch with a single action potential. Typically, extracellular recordings indicate that fast motor neurons generate larger action potentials. This neuromuscular arrangement allows one to use electromyogram (EMG) electrodes to record muscle activity extracellularly and often to know exactly which motor neuron is observed.
The leg movements that occur during walking on a horizontal surface are associated with typical patterns of motor activity. In both the middle and hind legs of cockroaches, CTr extension is generated by a burst of activity in the slow depressor motor neuron (Ds). At faster speeds, one or more muscle potentials from the fast depressor motor neuron (Df) occur at specific times in the leg cycle. These actions generate the stance phase of the leg cycle and alternate with activity from several flexor motor neurons that produce the swing phase. The simultaneous extension at the FTi joint is generated by the slow extensor of the tibia (SETi) motor neuron and the fast extensor of the tibia (FETi), and is again opposed by activity in several flexor motor neurons that generate the swing phase of that joint. As expected, the motor patterns of the front legs are more complicated, matching the joint movements described earlier.
The cockroach controls the speed of walking by altering the frequency of motor action potentials. Motor frequency is positively correlated with stepping frequency and joint velocity. Thus, the animal can move faster by increasing the frequency of Ds and SETi in each leg. It can turn by increasing frequency in one or more legs of the tripod while decreasing the frequency in the leg or legs located on the opposite side. This change creates stronger forces on the outer leg of the turn and weaker lateral forces on the inner leg, thereby turning the animal.
PATTERN GENERATION
Although both stance and swing phase motor neurons increase burst duration with increasing walking speed, the change in stance phase motor activity is much greater. This observation led to a model for insect walking referred to as the flexor burst generator. In this model, a set of interneurons within the central nervous system called a central pattern generator controls the flexor motor neurons directly and the stance phase motor neurons indirectly through inhibition from swing phase interneurons.
Investigators have recorded traces from oscillatory neurons in cockroaches and stick insects that could be part of such a central pattern generation circuit. For example, in the cockroach, neurons have been found that undergo membrane potential oscillations in time with levator motor activity. Moreover, stimulation of these cells activates the flexor motor neurons. Thus, these cells could be part of the flexor burst pattern generator. However, they by no means represent the entire circuit.
Observations in stick insects strongly suggest that there are separate burst generators that activate motor neurons for each joint in the leg. Patterned activity can be recorded in motor neurons for each joint of a single leg even after removing all sensory inputs. However, the timing of oscillations is not correlated between joints. Hence, the notion of separate controls. How then are these separate oscillators coordinated during walking, when, as noted above, CTr and FTi joints often move in perfect harmony? To answer this question, we must first understand something about the contribution of leg sensors to walking patterns.
LEG SENSORS
Even where central pattern generation circuits contribute to the control of locomotion, sensory systems still play important roles in generating behaviorally meaningful motor activity. This concept was particularly well demonstrated in the locust flight system. Locust preparations that have been isolated from sensory structures in the periphery (deafferented) are capable of generating bursts of activity that could move the wings in an appropriate manner. However, the frequency of the “wingbeat” cycle is much lower than normal and the pattern quickly dies out, whereas in the intact animal, flight can go on for very long periods of time. If appropriately timed activity from wing stretch receptors is added in, a more normal flight pattern occurs that is maintained for long periods of time. Thus, the central circuits are capable of generating part of the cyclical pattern but not a complete, behaviorally relevant one.
Similarly, normal walking behaviors in all insects that have been studied require input from sensory structures on the legs. However, in rapid running movements associated with escape, feedback from sensory inputs probably is too slow to influence walking within a single step, and the animal may be running “on autopilot.”
The sensors that play an important role in walking provide precise bits of feedback information to the walking system through structures that are most often found in the same locations in all three pairs of legs (Fig. 2). Internal sensors such as the chordotonal organs span the joints and monitor joint angle. Hair plates (HP) are positioned near joints where adjacent segments will touch during maximal flexion. Campaniform sensilla (CS) are located in strategic locations near joints and muscle insertions and detect strain in
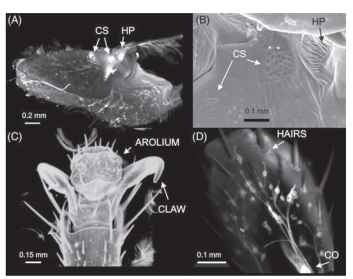
FIGURE 2 Sensory and cuticular structures of cockroach leg.
(A) View of the trochanter in a confocal microscope in which the main leg nerve was infiltrated with fluorescent dye (diI) that diffuses in membranes and stains sensory neurons when it is applied to peripheral nerves. The sensory neurons of campaniform sensilla (CS) and the hair plate (HP) are filled with the dye and fluoresce brightly.
(B) Scanning electron micrograph of the region containing the sense organs, showing cuticular caps of campaniform sensilla and long hairs of a hair plate. (C) Scanning electron micrograph of the distal end of the tarsus (foot), showing the hooklike claws and the arolium (an adhesive pad between the claws). (D) View of the dorsal side of the claw shows numerous sensory neurons stained with diI that innervate hairs as well as a portion of the chordotonal organ (CO). [Photomicrographs (A) and (D) by Faith Frazier, (B) by David Neff, and (C) by Laura Quimby, Marshall University School of Medicine.] the cuticle. These structures are made up of sensory nerve endings positioned in sockets with a flexible dome. As strain increases in the surrounding cuticle, the dome is deformed and activates the sensory neuron. There are groups of campaniform sensilla near each of the leg joints, including the segments of the tarsus.
Each peripheral sensor provides information that is used at discrete phases of the step cycle as the insect walks. Some sensory inputs in walking are essential in making transitions between the stance and swing phases while others adapt muscle activities to reach levels needed for support and propulsion. The effects of leg sense organs are demonstrated particularly well in a computer simulation that was developed by Ekeberg, Bumel, and Buschges for stick insect walking. The simulation is controlled by calculations similar to those done by the nervous system. Signals from chordotonal organs that monitor joint angles are used to determine the times of transition from swing to stance, when the leg is first placed on the ground. Inputs from campaniform sensilla that detect load aid in generating extensor muscle activities in a self-regenerating loop known as positive feedback. Extensor activities continue until chordotonal inputs trigger the start of swing.
Beyond helping to set the normal locomotor pattern, these sensors are, of course, readily available to make rapid adjustments to the motor pattern as the animal moves away from horizontal surfaces and climbs inclines or vertical surfaces, or even walks on ceilings and negotiates obstacles in its path. Recent experiments have shown that the tibial campaniform sensilla can aid in this process by detecting the proportion of body weight that is supported by a leg. In these studies, load was varied by using magnets attached to the body and an electrical coil under the floor on which the insect stood. Some campaniform sensilla discharged when the load was increased while others fired when the body load was decreased. Those signals could be important in detecting when a leg slips.
Arrays of neurons with diverse properties receive sensory inputs and could mediate appropriate effects. Within each thoracic ganglion, the sensory neurons from these leg sensors project to populations of spiking local interneurons (interneurons that are isolated to a single ganglion). The projections of many receptors follow a topographic pattern that reflects the location of the sensors in the leg. The spiking interneurons project both to motor neurons and to another set of local interneurons called nonspiking interneurons. These interneu-rons never generate action potentials. Rather, they act through graded potentials to control and coordinate motor activity to various leg muscles within a thoracic segment. Coordination among joints of each leg (intraleg coordination) occurs through interactions in these local circuits.
Finally, spiking interganglionic interneurons project between thoracic ganglia, influencing local circuits for adjacent legs. Through these circuits, the sensory activity directs appropriate adjustments in individual muscles, coordinates segments within a single leg, and influences the coordination between legs.
The notion of feedback circuits influencing timing of leg movements suggests a solution to coupling of joint pattern generators alluded to earlier. Leg sensors influence timing of extension both in the motor neurons that control the joint that is monitored and in motor neurons serving adjacent joints. These weaker connections provide coupling for the joint pattern generators. Moreover, that coupling can be altered to provide for considerable flexibility. In this way, the joint pattern generators that produce walking could also be used in other rhythmic behaviors, such as righting (when an animal falls over and uses its legs to regain an upright posture), turning, or grooming, by changing the coupling between the oscillators. Indeed,in the cockroach, reflex effects in adjacent leg joints can be reversed by altering signals descending from the brain.
In stick insects, the influences of some sense organs are changed when the direction of walking is reversed. Stick insects readily walk forward or backward, as is needed when negotiating branches of trees. However, backward walking involves changing the activities of leg muscles. For example, protractor muscles are active in swing in forward walking but fire during stance when the animal walks backward. The effects of load-detecting sense organs on motor activities also change when the animal reverses direction. Trochanteral sensilla that excite the retractor in forward walking instead activate the rec-tractor when the animal walks backward. The nervous system, therefore, changes the effects of the sense organs so that they counter the perturbation in the direction in which the animal is walking.
INTERLEG COORDINATION
To generate an effective tripod gait, insects must coordinate movements of joints not only within a single leg but also between legs. This interleg coordination on the surface appears to be a daunting task. The tarsi of the three legs that make up an effective tripod must move in synchrony. However, as described earlier, the legs of each segment make different movements. The patterns of the motor neurons that control those movements also differ in each leg. Thus, the animal must coordinate the tarsi through joint movements and neural activities that are specific to each pair of legs.
Observations on stick insects suggest that these potentially complex connections can be functionally formulated as a set of fairly straightforward rules indicating that events in one leg that are readily detected by leg sensors influence the actions of other legs in discrete ways. For example, one rule is that when one leg is in swing, the adjacent anterior leg cannot enter into swing phase. Alternatively, the start of the stance phase promotes the start of swing in adjacent anterior legs and in the contralateral leg of the same segment. More subtle influences also exist to account for transient changes. For example, an increased load on one leg prolongs the power stroke on the con-tralateral leg of the same segment. This would result in coactivation of legs when the animal is encountering increased resistance, such as during climbing. The specific neural connections that underlie these rules are the focus of an exciting new area of research.
NEGOTIATING OBSTACLES
The remarkable agility that attracts interest from roboticists is seen when insects face an obstacle. Most insects effortlessly negotiate barriers that would pose considerable control problems in robots. This problem is only now beginning to be investigated, because climbing over obstacles is a more transient event than horizontal walking, and there is considerable variability in the strategies used. However, some common themes are evident. In climbing over a block, for example, insects must move their CoM upward to surmount the obstacle. For cockroaches, the strategies used to accomplish this task vary with the size of the obstacle and the speed that the insect is moving at when the block is encountered. The front legs are normally lifted up fairly high during normal walking. If the block that is encountered is lower than the normal front leg trajectory, the insect hardly needs to change its movement at all. The front leg will be placed on top of the obstacle and pushed down to move the animal’s CoM upward as a natural consequence of the encounter with the block. Somewhat larger barriers can be negotiated in this way when the insect is moving at faster speeds.
Larger blocks, especially at slower speeds, require a redirection of leg movements that now push the body upward upon joint extension. This rearing movement allows the front legs to be readily placed on top of the block so that climbing can commence.
These redirected leg movements often require intervention from descending activity originating in the brain. Lesion of a region of the brain called the central complex (CC) compromises the cockroach’s ability to negotiate barriers. Although little is known regarding how the CC generates appropriate descending commands, neurons in this region receive sensory inputs from a variety of structures including mechanoreceptors on the antennae and visual systems.
For very large obstacles, the insect must actually climb up the front face of the object. Here the insect must attach itself as if the face of the block were a wall. This problem is solved by a combination of claws and pads that adhere to various substrates. Cockroach claws are normally held up during horizontal walking, like the claws of a cat. However, when a cockroach is walking up a vertical surface, the claw is pulled down by a muscle in the tibia, which attaches to the claw through a long apodeme. There is no return muscle. Rather, as we have described, the claw pops back up passively with the aid of strategically placed resilin ligaments. For smoother surfaces, including those as polished as glass, some cockroaches have cuticular pads between their claws that can adhere to the substrate for stability but be readily lifted when needed in walking.
Many insects have developed efficient jumping behaviors to move long distances with a single jump. The jump requires a powerful and rapidly accelerating movement of the jumping leg. Orthopterans such as grasshoppers and locusts have very large hind legs that can generate powerful jumping movements. However, even the large femur extensor muscles of these jumping legs cannot generate the quick extension necessary for an efficient jump without some mechanical modification within the leg structure for storing energy. In locusts, the tendon of the tibial flexor muscle moves over a stop that allows the extensor to contract without moving the leg when the muscles are coactivated. As a result, a considerable amount of energy is stored in the mechanical distortion of the femur, tibia, and extensor tendon (like the bow of an archer). Inhibition of the tibial flexor muscle releases locking mechanisms and produces a very rapid and powerful extension that propels the animal upward. The timing of these events is critical, and neural circuits have been identified in association with kicking movements that provide the appropriate control.
Fleas generate remarkable jumps relative to their tiny size. Again, a proportionally very large hind leg is used to generate the jumping movement, with a hook on the hind leg preventing movement until a large isometric force has been achieved. The isometric force is stored in strategically located resilin ligaments and released during the jump.
One of the most remarkable jumping strategies in insects does not even involve legs. Click beetles can jump from a standstill to four times their body length by rapidly accelerating the joint between the prothoracic and mesothoracic segments of the body. Here again a mechanical stop prevents movement until a large isometric force has been achieved, this time in thoracic muscles, and then is released suddenly to shoot the animal upward.
ROBOTIC DEVICES INSPIRED BY INSECT WALKING
The efficiency and agility of walking insects as well as specialized strategies such as jumping have not been lost on robotics engineers. Insects have provided popular models for legged robots because the hexapod gait is statically stable. That is, the CoM remains within the base of support of at least one tripod at all times. Thus, the control for a robot need not actively maintain balance during horizontal walking. However, to capture the agility of the insects, postural adjustments, reflexes, efficient leg design, and sensors must be incorporated into the robotic designs. This realization has generated collaborations between engineers and biologists to study insects and then incorporate newly discovered control and mechanical properties into more efficient robots. The efforts to develop and control these new robots in turn provide new insights into how the insect controls its own movements.

