Ticks
Ticks comprise a distinct group of exclusively blood-feeding ectoparasites familiar to most people in virtually all regions of the world. Ticks transmit a greater variety of disease-causing pathogenic agents than any group of arthropods, including protozoan, viral, bacterial, and even fungal pathogens. An example is Lyme disease (LD), which is now the most important vector-borne disease of humans in the United States, Europe, and Asia. In numerous countries in tropical and subtropical regions of the world, tick-borne diseases of livestock such as babesiosis, theileriosis, and heartwater have made it difficult or impossible to raise domestic animals for food or animal products. Ticks can also cause irritating or even fatal injury to humans and animals because of paralysis, toxicity, or severe allergic reactions to their bites.
BODY STRUCTURE
The tick body is organized into two major body regions, the anterior capitulum, bearing the mouthparts, and the idiosoma, bearing the four pairs of walking legs (Figs. 1 and 2 ). There is no head, and

FIGURE 1 Scanning electron micrographs of a representative adult female ixodid tick, D. variabilis: (A) dorsal view and (B) ventral view. Scale = 1mm.
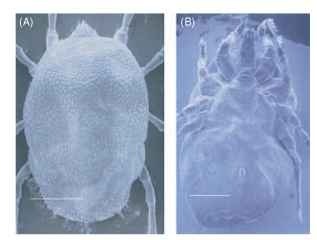
FIGURE 2 Scanning electron micrographs of a representative adult female argasid tick, O. parkeri: (A) dorsal view and (B) ventral view. Scale = 1 mm.
the highly fused body is not divided into a thorax and an abdomen. The capitulum contains the toothed hypostome, which embeds the tick into the host’s skin and also contains the food canal for blood imbibition, the chelicerae, delicate paired appendages that cut into the skin, and the four-segmented paired palps that provide important sensory information for host identification. In argasid ticks, the capitulum is recessed under an anterior extension of the body. The remainder of the body contains the genital pore, anus, and spiracles, which are visible on the ventral surface. In ixodid ticks (so-called hard ticks), a prominent platelike scutum is found on the dorsal surface. Argasid ticks (so-called soft ticks) are similar to the ixodid ticks but lack a scutum, and the body cuticle is leathery.
The interior of the tick body is a simple, open cavity called the hemocoel that is filled with a circulating fluid, the hemolymph, which bathes the internal organs. In ticks, as in other terrestrial arthropods, there is no hemoglobin, and the hemolymph does not function in oxygen transport. Most of the body interior is occupied by the mid-gut, the largest internal organ of the tick body, which consists of a central saclike stomach and several lateral diverticuli. Other prominent internal organs are the paired salivary glands, which appear as white grapelike clusters, and the reproductive organs. In females, these are the ovary, paired oviducts, uterus, and seminal receptacle, and the vagina that connects the system to the genital pore. During feeding, the ovary enlarges and becomes distended with large, brown or amber-colored eggs. In males, the reproductive system consists of the testis, vasa deferentia, and seminal vesicle, and the ejaculatory duct, which is connected to the genital pore. Much of the system is obscured by the large, white multilobed accessory gland. This gland provides the components for the saclike spermatophore that the male tick uses to transfer its sperm to the female. Also present are numerous tracheal tubes, connected to the marginal spiracles, that provide the respiratory system, and the Malpighian tubules and rectal sac, connected to the anal pore, for waste elimination. Argasid ticks have a pair of coxal glands that excrete via the coxal pores excess water and salts accumulated during feeding. The fused central nervous system, the synganglion, is located in the body above the genital pore.
SYSTEMATIC RELATIONSHIPS
Ticks are classified with the class Arachnida, the group that contains the familiar spiders and scorpions. Arachnids have chelicerae, which are appendages with pincerlike or scissorlike cutting edges, instead of mandibles. There is no head or thorax such as occurs in insects. There are no antennae. Ticks are grouped together with the mites in the subclass Acari. Ticks constitute a distinct suborder, the Ixodida, within the acarine order Parasitiformes. The Ixodida contains three families—the Ixodidae, Argasidae, and Nuttalliellidae. The Ixodidae or hard ticks are by far the largest of the different families of ticks, with approximately 650 species. Ixodid ticks have three active life stages, including a single nymphal stage. Hard ticks contain most of the important disease vectors and pest species that plague livestock and wildlife. The Ixodidae are further subdivided into the Prostriata, represented by the single genus Ixodes, which is easily recognized by the anterior anal groove, and the Metastriata, which include the remaining 13 genera, in which the anal groove is posterior to the anal aperture. The Ixodidae include the ticks that transmit the agents of LD, Rocky Mountain spotted fever (RMSF), boutonneuse fever, babesiosis and theileriosis of livestock, and most of the other tick-borne disease-causing agents.
The Argasidae comprise the soft ticks, with their leathery, highly flexible cuticle. There are approximately 170 species divided into five genera (four according to some authorities). Soft ticks also have three active life stages, but most species have multiple nymphal stages before they develop into adults. Ticks of the genus Ornithodoros (>100 species) have a leathery cuticle with innumerable small elevations known as mammillae and a rounded body margin. Ticks of the genus Argas (~58 species) have a flattened lateral margin marked by a sutural line. The leathery cuticle bears small ridges and folds in a rectangular pattern, each with a pit at the center of these buttonlike enclosures. Except for species that transmit the spirochetes that cause relapsing fever, most soft ticks are not important in the transmission of disease.
The third family of ticks is the monospecific family NuttallieUidae, represented by only one species, Nuttalliella namaqua) in southern Africa. It contains structures characteristic of both other tick families. Its highly wrinkled cuticle with pits and elevations resembles the cuticle of argasid ticks, but its dorsal pseudoscutum resembles the scutum of the ixodid ticks.
Ticks are an ancient group of specialized acarines that were already well developed during the Mesozoic era (i.e., the era of the dinosaurs). A larval tick found in amber in New Jersey was dated between 90 and 94 mya (i.e., during the Upper Cretaceous period). Although exhibiting some unusual characteristics in its setal arrangements, the tick was readily characterized as a member of the genus Carios, a genus that exists today. This finding suggests that this genus at least (and perhaps other argasid ticks) has not changed very much in many millions of years.
TICK BIOLOGY: LIFE CYCLES, FEEDING
BEHAVIOR, DEVELOPMENT, AND REPRODUCTION
The tick life cycle comprises the egg and three active stages, namely, larva, nymph, and adult. There is only a single nymphal instar in the ixodid tick life cycle, but varying numbers of nymphal stages may occur in the argasid tick life cycles. All ticks feed on blood during some or all stages. Most species are three-host ticks; that is, each stage attacks hosts, feeds, and detaches before developing into the next life cycle stage. Adult ticks seek hosts, feed, and, in the case of engorged females, drop off to lay their eggs. Ticks can survive for long periods between blood meals. Consequently, when feeding is delayed, the life cycle may be extended for years or, in the case of some argasids, for a decade or longer. There are major differences between the life cycles of the Ixodidae and the Argasidae, as discussed in the following sections.
Life Cycles of Ixodid Ticks
The ixodid ticks feed slowly, from several days to as long as 2 weeks. Immature and adult ticks each take a blood meal, except for the nonfeeding males of some species. After crawling onto their hosts, these ticks embed their mouthparts into the host skin and secrete cement from their salivary glands into and around the wound site to anchor themselves. The cement binds the ticks firmly in place and makes them very difficult to remove. During blood feeding, the ticks secrete potent anticoagulants and anti-inflammatory agents, which suppress host wound healing and facilitate blood flow. As the ticks feed, new cuticle is synthesized to accommodate the enormous blood meals the animals consume, often 10-100 times their original body weight. Females feed only once. Mating occurs during feeding, although ticks of the genus Ixodes may also mate prior to host attachment. In the metastriate Ixodidae (i.e., ixodids other than ticks of the genus Ixodes), mating occurs within a few days after the commencement of feeding and is regulated by sex pheromones, including the volatile 2,6-dichlorophenol and the nonvolatile cholesteryl esters on the body surface. Following mating, females suck blood rapidly (24-48 h) and swell enormously, whereupon the replete females drop from their hosts, find a sheltered location, and lay thousands of eggs. An example is the American dog tick, Dermacentor variabilis, which typically lays more than 5000 eggs. Following oviposition, the female dies. In contrast to the females, males swell only slightly during feeding. However, they can mate many times, feeding between matings. In certain species of Ixodes . mating may occur either on or off the host (e.g., the blacklegged tick, Ixodes scapularis. or the sheep tick, I. ricinus). In some nest-inhabiting Ixodes species, the males have vestigial hypostomes. These ticks always mate off the host.
Once oviposition has been completed, the larvae hatch in the thousands and begin host-seeking activity. Except for the nest-inhabiting species, the larvae disperse into the vegetation, where they come in contact with passing animals. Once they have attached to a host, the larvae embed themselves into the host skin, form a feeding pool, and engorge in the manner already described. Feeding usually takes 2-4 days, whereupon the engorged larvae drop from their hosts to molt on the ground. Molting usually occurs in some sheltered microhabitat such as soil or leaf litter, or in host nests. After molting, nymphal and adult ticks must seek another host and feed. However, more than 90% of the life cycle is spent off the host. When host seeking and feeding occur in all three parasitic stages, the pattern is termed a three-host life cycle.
A few ixodid species exhibit a two-host or one-host life cycle. For example, in the camel tick Hyalomma dromedarii, both larvae and nymphs feed on the same host (two-host life cycle), and in the cattle tick Boophilus annulatus, all stages feed, molt, and even mate on the same host (one-host life cycle).
Life Cycles of Argasid Ticks
Feeding is very rapid among the argosid ticks. Once they have crawled onto a host, the ticks embed their mouthparts in the same manner as their ixodid relatives, but without secreting cement. Bloodsucking commences quickly and, as feeding progresses, the bloated ticks excrete copious quantities of a clear, colorless coxal fluid (sometimes this occurs soon after feeding). By eliminating excess water and salts via the coxal fluid, the ticks can concentrate their blood meals and adjust their internal water balance. The ticks expand to about 5-10 times their original body weight, depending on the ability of the cuticle to stretch. Following feeding, often completed within as little as 30-60 min, the replete ticks drop off to molt or, if female, to lay eggs. Argasid females take repeated small blood meals and lay small batches of eggs (typically <500 eggs in a batch) after each feeding (multiple gonotrophic cycles). The interval between feedings is typically several months but may be up to several years, depending on host availability. Mating usually occurs off the host. Because of the multiple nymphal instars (six or even seven in some species), argasid ticks often live for many years. In addition, these ticks are highly resistant to starvation, an advantage that can extend their longevity even further. As a result, the entire life cycle may take from 10 to 20 years.
Following oviposition and hatching, most argasid tick larvae seek hosts, feed rapidly, and molt to the first nymphal instar. These nymphs seek hosts again, feed rapidly, and molt to the second nymphal instar. Subsequently, the life cycle varies considerably, leading to additional nymphal instars or proceeding directly to the adult stage. As a rule, males emerge earlier than females and have fewer nymphal stages. In some argasids, especially bat parasites, the larvae remain attached to their hosts for many days, feeding slowly, just like their ixodid tick relatives, and then molt twice without additional feeding. Thereafter, the life cycle resembles the typical argasid pattern. Another unusual species is Otobius megnini, which has only a single nymphal stage. Neither the males nor the females feed, and the females lay eggs without having had a blood meal (i.e., autogeny).
ECOLOGY
Most ticks are exophiles (i.e., nonnidicolous, living exposed in the open environment rather than in shelters). Most ticks live in forests, savannahs, brush, grassy meadows, or under stones, crevices, or even in sand in semidesert environments. Others, however, are nidicoles, surviving in caves, burrows, houses, cracks, and crevices where their hosts obtain shelter. This habit is characteristic of most argasids and many species of the genus Ixodes.
Seasonal Activity and Host-Seeking Behavior
Exophilous ticks are active during certain periods of the year when climatic conditions are suitable for development and reproduction. During this seasonal activity period, they attack and feed on suitable animals. This is known as host-seeking behavior. At other times, ticks remain in diapause (i.e., a state of reduced metabolic activity). In temperate and subpolar regions, the seasonal activity period is regulated by ambient temperature, changing photoperiod, and incident solar energy. Tick seasonal activity usually commences with the onset of warmer weather and increasing daylength. In what is termed the ambush strategy, hungry ticks climb on the vegetation to varying heights, depending on life stage (e.g., adults climb the highest) and cling to any passing animals. In some species, the ticks emerge from their shelters and run toward their hosts when they detect animal odors (or, rarely, noise) from animals nearby. This so-called hunter strategy is useful in arid habitats, where there is little vegetation or source of other moist, protective covering. Argasid ticks normally do not exhibit seasonal activity, since they live in proximity to their hosts in nests, burrows, or other shelters. However, in some species specific for migratory birds or bats, host-seeking activity is synchronized with the period of the year when these hosts return to reoc-cupy their nests.
For many ixodid ticks that occur in temperate or subarctic regions, seasonal activity begins in the spring. In D. variabilis, for example, larvae that survived the winter begin to feed on small mammals. Activity accelerates rapidly as increasing numbers of larvae, stimulated by rising soil temperatures and lengthening photoperiods, emerge to attack these animals, reaching the seasonal peak within a few weeks. Feeding by nymphal and adult ticks follows soon afterward, with the adult peak in early summer. In the southern parts of its range, the tick’s entire life cycle, from eggs to ovipositing females, is completed in 1 year. In the northern parts of the D. variabilis range, larval emergence is delayed until late spring. Moreover, the cooler soil temperatures and shorter daylengths delay molting of fed ticks. As a result, adults emerge from fed nymphs in late summer or early fall, when soil temperatures and incident solar radiation are declining, and this results in a 2-year life cycle. Thus, both a 1-year and a 2-year life cycle can occur because of variations in climatic conditions within this wide-ranging species.
In I. scapularis, larvae and nymphs feed in the spring and summer, as in D. variabilis, whereas adults are active in the fall and early spring. However, the order of larval and nymphal feeding is the opposite of the dog tick. Nymphal ticks emerge from their overwintering diapause in spring or early summer, depending on the region of the United States where they occur. Larvae appear next, typically a month or two after the nymphal peak. Meanwhile, fed nymphs molt over the summer, but the newly emerged adults delay host-seeking activity until the cooler months of the fall or early winter. This life cycle pattern enables nymphs infected with Borellia burgdorferi to infect mice, providing thereby a reservoir of infected hosts to infect the next generation of larval ticks. The implications of these different tick life cycle patterns for the survival and transmission of zoonotic diseases are discussed further in the sections on specific diseases.
Occasionally, ticks are active only during the winter months. An example is the winter tick, D. albipictus, a one-host tick that feeds on horses, deer, elk, moose, and other large ungulates. In this case, larvae commence host-seeking activity in late summer or early fall. Larvae and nymphs feed and molt on the same hosts and the resulting adults reattach, feed, and mate. Feeding and development require many weeks for completion, even though these activities occur on the same host and, as a result, the adults are often found on their ungulate hosts in winter or early spring. In D. albipictus, declining photoperiod stimulates feeding activity, just the opposite of the pattern seen in D. variabilis.
Host Specificity
Host-seeking activity is strongly influenced by the availability of hosts and host selection behavior. All ticks species exhibit varying degrees of host specificity; most (>85%) exhibit relatively strict host specificity. At one extreme are the argasid ticks that feed exclusively on bats (e.g., ticks of the genus Antricola and certain species of Ornithodoros). For example, during his graduate student years, Sonenshine colonized the bat tick, Ornithodoros kelleyi, in the laboratory. To feed the ticks, he had to maintain a colony of bats collected from limestone caves or attics of old buildings. No other hosts would do. Similarly, the cattle ticks Boophilus microplus and B. annulatus feed solely on cattle and, when available, on white-tailed deer. Other species exhibit limited host specificity (e.g., D. variabilis). Larvae and nymphs of D. variabilis feed on a wide range of small mammals (e.g., white-footed mice and meadow voles), but never on carnivores, ungulates, humans, or other large mammals. In contrast, adults of this species feed on medium-sized and large mammals, including humans (although they can be induced to feed on rodents when confined in capsules). Finally, at the opposite extreme of the specificity spectrum are the opportunistic species that feed on hosts of virtually all types (e.g., I. scapularis and I. ricinus). Immatures feed on lizards, birds, and small, medium-sized, and large mammals, including deer and humans. Adults feed on medium-sized and large mammals, including humans. Although the range of confirmed hosts is astounding, these opportunistic ticks have preferred hosts (e.g., mice for the immatures; deer, sheep, and other mammals for the adults). Host specificity is also strongly influenced by ecological adaptations, so that ticks adapted to a particular habitat in a given region of the world will encounter only vertebrates adapted to the same habitat.
As tick-host associations evolved, ticks gradually developed the ability to facilitate long-term feeding by evading or suppressing host homeostatic systems. For example, I. scapularis saliva contains pharmacologically active compounds that suppress edema and inflammation in their hosts while enhancing vasodilation. This leads to greater blood flow into the wound site without the pain and intense itching sensation so characteristic of the bites of mosquitoes or biting flies. These adaptations are most effective for the hosts encountered most frequently by each tick species, so-called preferred hosts, but less effective for uncommon hosts.
Survival between Blood Meals
One of the most remarkable aspects of tick biology is the ability to survive for long periods between blood meals. The tick’s midgut serves as a storage organ where the blood meal is digested slowly over long periods. Among the argasids, individuals may survive for several years without feeding while waiting for the occasional wandering hosts that enter their secluded shelters. Among the exophil-ous ixodids, survival periods are much shorter, but even these ticks may survive for up to 1 year between blood meals. According to a study by Needham and Teel in 1991, ticks spend more than 90% of their life history off the host.
Ticks must also conserve body water to survive while they wait for hosts. While questing (i.e., perching for attack) on short stems, blades of grass, or other vegetation, ticks are exposed to desiccating conditions that can become life threatening within a few days or weeks, depending on the species. Among the desiccation-intolerant I. scapularis and I. ricinus, which are adapted to cool, humid forest habitats, desiccated individuals retreat to the forest floor or rotting vegetation at the base of a meadow. In these nearly saturated humid microenvironments, they can restore their water balance by a process known as atmospheric sorption, in which the partially desiccated ticks salivate salt-rich secretions onto their hypostomes. This hygroscopic secretion collects moisture, which is sucked back into the body. Since, however, the process demands a considerable expenditure of energy, the number of cycles of desiccation and sorp-tion is limited as the tick’s age. Other species, such as the relatively desiccation-tolerant Hyalomma asiasticum, can survive for long periods in the semidesert habitats in central Asia, where it waits for passing camels and other large ungulates. Nidicolous ticks, sheltering in caves, burrows, or other protected microenvironments, are subject to less stressful conditions during the long wait between hosts. These ticks exhibit behavioral patterns that restrict their distribution to these sheltered locations.
REPRESENTATIVE TICK-BORNE DISEASES
In view of the exceptionally large variety of diseases caused by tick-borne pathogens and injurious substances, this section is limited to a brief description of several representative examples, with primary emphasis on development of the infection in the tick and tick vector ecology. For a more extensive review, the reader may wish to consult topics by Sonenshine and Strickland, or review articles, for information on the specific diseases. Table I lists some representative tick-borne diseases affecting humans and animals.
Lyme Disease
The most common tick-borne disease affecting human health in the world today, LD, occurs throughout most of the United States and southern Canada, Europe, and northern Asia. The disease is caused by B. burgdorferi (sensu latu), a type of bacterium known as a spirochete. B. burgdorferi is the causative agent of LD in most of the United States. A second genospecies, B. lonestari, was isolated from lone star ticks in the southeastern United States, but the relationship of these spirochetes to LD in humans is uncertain. In Europe, LD is caused by B. afzellii and B. garinii as well as B. burgdorferi.
In humans, LD results from the bite of an infected tick, either a nymph or adult of the genus Ixodes. The tick must have remained attached for several days to allow for the bacteria to travel from the tick’s midgut to its salivary glands and into the wound site. Symptoms begin several days to several weeks later. Onset of illness is characterized by mild, flulike fever and, in most patients, a reddish skin rash, known as the erythema migrans (EM). The typical EM rash is a gradually expanding circular or elliptical lesion with a red margin and clear center, at least 5 cm or more in diameter, and often near the site of the tick bite. Some patients show multiple EM rashes. If left untreated in this early stage, the fever abates, the rash fades, and the patient may recover without any further symptoms. Often, however, the bacteria remain in people’s bodies and spread into the nervous system and joints, where they cause the long-lasting secondary symptoms of chronic LD. Late manifestations include arthritis, especially in the knee joints, which frequently spreads to different joints (migratory polyarthritis). Damage to the synovial membranes is a distinctive feature of this type of arthritis because of invasion of the synovial fluid by the spirochetes. Neurological symptoms include nerve pain (peripheral neuropathy), various types of palsy caused by nerve damage (e.g., Bell’s palsy), and central nervous system disorders. Many patients suffering from some or all of these symptoms simultaneously also complain of severe fatigue. In Europe, another chronic feature of late LD is acrodermatitis chronica atrophicans, a condition in which the skin atrophies and peels. Chronic LD is greatly feared in regions where it is endemic because, among other reasons, it can persist for many years despite treatment with antibiotics.
Only a limited variety of ticks of the genus Ixodes are competent vectors of LD spirochetes. Other biting arthropods may acquire these bacteria, but they cannot transmit the bacteria when they feed again. In the eastern and central United States, the only proven vector to humans is I. scapularis. In the western part of the country the vector is the western blacklegged tick I. pacificus. However, other species of the so-called I. ricinus complex that feed solely on wildlife contribute to maintaining the disease in nature; examples include I. spinipalpis (I. neotomae) in the west and I. dentatus in the east. The sheep tick, I. ricinus, is the primary vector in Europe and western Asia, while the taiga tick, I. persulcatus, is the primary vector from eastern Europe across most of northern Asia. Other species of the I. ricinus complex (e.g., I. ovatus) also serve as efficient enzootic vectors.
The cycle of B. burgdorferi development in the tick begins with ingestion of an infectious blood meal by the larvae as they feed on a mouse or other reservoir host. Spirochetes survive in the midgut diverticula but are not disseminated until the tick molts to the nym-phal stage and feeds again. Following the influx of fresh host blood, the spirochetes surviving in the midgut pass between the cells of the gut wall and disseminate to the salivary glands and other internal organs. However, in females, spirochetal invasion of the ovary damages the developing oocytes, which prevents efficient transovarial transmission. As a result, transmission is by the transstadial route. As the tick feeds, spirochetes escape with the saliva and are introduced to the wound site of the new host. Transmission from nymph to adult is also common.
In the eastern United States, the risk of acquiring LD is greatest in the late spring or early summer because of the peculiar nature of the LD cycle in nature. Nymphal ticks infected with B. burgdorferi emerge from their overwintering diapause to attack hosts, especially white-footed mice and other small mammals, as well as humans. This feeding pattern ensures the presence soon afterward of numerous infected hosts when the larvae emerge, spreading the B. burgdorferi infection to vast numbers of these tiny ticks. Fed larvae molt in late summer or early fall, but the resultant nymphs diapause rather than commence feeding. Thus, the infection is perpetuated from one year to the next. Meanwhile, adults that emerge from fed nymphs delay feeding until the fall, whereupon they seek white-tailed deer and other large mammals (including humans). This pattern results in a 2-year cycle throughout most of the tick’s geographic range.
Although the vector ticks are opportunistic feeders and will attack most vertebrates, only a limited variety of hosts are competent reservoirs of B. burgdorferi. Only competent reservoirs, animals capable of maintaining spirochetes within their tissues for prolonged periods, can infect ticks that feed on them. Examples of competent reservoirs include the white-footed mouse, the dusky-footed wood rat, the California kangaroo rat, and several other rodents in North America, and such animals as the common shrew, bank vole, wood mouse, yellow-necked field mouse, and hares and pheasants in Europe. Several species of birds are also reservoir competent and play an important enzootiologic role by dispersing infected ticks over considerable distances, thereby establishing new foci of infection. In contrast, some of the animals that are excellent hosts of vector ticks, such as white-tailed deer and western fence lizards, destroy invading spirochetes and, therefore, are not reservoirs. Such animals serve as amplifying
TABLE I |
||||
Representative Tick-Borne Diseases, Their Causative Agents, Tick Vectors, and Reservoir Hosts |
||||
| Disease | Causative agent | Primary tick vector species | Affected hosts | Major clinical symptoms |
| Protozoan | ||||
| Human babesiosis | Babesia microti, B. | Ixodes scapularis, I. ricinus | Humans | Malaria-like fevers, myalgia, arthralgia, |
| divergens, B. gibsoni | nausea, sweating | |||
| Bovine babesiosis | B. bigemina | Boophilus annulatus, B. | Cattle | Hemoglobinuria (redwater) fever, death |
| microplus, others | ||||
| East Coast fever | Theileria parva | Rhipicephalus | Cattle, buffalo | Fever, lymphadenopathy, pulmonary edema |
| appendiculatus | ||||
| Tropical theileriosis | T. annulata | Hyalomma anatolicum | Cattle, horses | Fever, lymphodenopathy, pulmonary edema |
| Feline cytauxzoonosis | Cytauxzoon felis | Dermacentor variabilis | Cats | Fever, emaciation, splenomegaly, death |
| Bacterial, extracellular | ||||
| Tularemia | Francisella tularensis | Haemaphysalis leporis- | Humans, various | Fever, headache, pustular, ulcerated papu- |
| palustria, other tick | other mammals | lae; pneumonia, pleuritis, rash; however | ||
| species | few deaths | |||
| Lyme disease | Borrelia burgdorferi, B. | Ixodes scapularis, I. ricinus, | Humans, dogs, cats, | Initial phase: fever, EM rash Chronic phase: |
| afzelii, B. garinii | I. pacijicus, I. persulca- | domestic animals | arthritis, neurologic symptoms | |
| tus, others | ||||
| Tick-borne relapsing | Borrelia spp. | Ornithodoros spp. | Humans | Intermittent fevers, chills, fatigue, myalgia, |
| fever | arthralgia; generally mild illness; death rare | |||
| Avian spirochetosis | B. anserina | Argas persicus | Birds | Fever, death |
| Epizootic bovine | Unknown, possibly B. | Ornithodoros coriaceus | Cattle, deer | Fever, spontaneous abortion |
| abortion | coriaceae | |||
| Bacterial, intracellular (Rickettsiales) | ||||
| Rocky Mountain fever | Rickettsia rickettsii | Dermacentar variabilis, D. | Humans | High fevers, spotted whole-body rash |
| andersoni, others | ||||
| Boutonneuse fevera | Rickettsia conorii | R. sanguineus, D. reticula- | Humans | High fever, rash, ulceration at bite site |
| tus, others | (eschar) | |||
| Human monocytic ehr- | Ehrlichia chaffeensis | Amblyomma americanum, | Humans | Fever, rash (sometimes), muscle aches, joint |
| lichiosis (HME) | D. variabilis | aches | ||
| Human granulocytic | Ehrlichia | Ixodes scapularis, I. pacifi- | Humans | Fever, rash (sometimes), muscle aches, joint |
| ehrlichiosis (HGE) | phagocytophilia | cus, I. ricinus | aches | |
| Tick-borne fever | E. phagocytophilia | I. ricinus | Sheep | Fever, weight loss, reduced milk production, |
| (sheep pyemia) | abortion | |||
| Canine ehrlichiosis | E. canis, E. ewingli, | R. sanguineus, I. ricinus, A. | Dogs | Fever, loss of appetite, weight loss, apathy, |
| E. phagocytophilia | americanum, others | death in severe cases | ||
| Heartwater | Cowdria ruminantium | Amblyomma hebraeum, A. | Ruminants | Fever, “pedaling behavior”, prostration, |
| variegatum, others | coma, death | |||
| Anaplasmosis | Anaplasma marginale, | D. andersoni, D. occidenta- | Cattle, sheep, other | Fever, anemia, death |
| A. centrale, A. ovis | lis, R. sanguineus, others | ruminants | ||
| Q fever | Coxiella burnettii | Many tick species | Humans, large | Low-grade fever, sweating, sore throat, |
| domestic livestock | pneumonia, severe frontal headache, | |||
| myalgia, photophobia | ||||
| Arboviruses | ||||
| Tick-borne encephalitis | Flavivirusb | I. ricinus, I. persulcatus | Humans, carnivores | Fever, headache, encephalitis, meningitis, |
| Flavivirusb | paralysis; death in severe cases | |||
| Powassan encephalitis | Ixodes, Dermacentor, | Rodents, hares, etc. | Fever, headache, encephalitis, neurological | |
| Haemaphysalis spp. | symptoms, brain damage, death | |||
| Colorado tick fever | Coltivirusc | D. andersoni | Rodents, humans, | Biphasic fever, headache, muscle aches, |
| Nairovirusd | domestic animals | joint pain | ||
| Crimean-Congo hem- | Hyalomma m. marginatum, | Hares, humans, | Fever, chills, headache, internal bleeding, | |
| orrhagic fever | H. m. rufipes, others | small mammals, | rashes; death in severe cases | |
| others | ||||
| Louping ill | Flavivirusb | Ixodes ricinus | Sheep | Fever, erratic, louping gait, loss of motor |
| encephalitis, death | ||||
| African swine fever | Iridovirus | Ornithodoros moubata | Domestic pigs, wild | Fever, internal damage; death in most cases |
| porcinus, O. erraticus | boars, warthogs | |||
| Tick-caused diseases | ||||
| Tick paralysis | Tick proteins | I. holocyclus, I. rubicundus, | Cattle, sheep, | Ascending paralysis, loss of motor control, |
| D. variabilis, D. ander- | humans, other | no fever; death | ||
| soni, others | mammals | |||
| Tick bite allergies | Tick proteins | Argas reflexus, O. cariaceus, | Humans | Nausea, vomiting, diarrhea, irregular pulse, |
| I. pacijicus, etc. | shocklike symptoms; rarely death | |||
| Sweating sickness (and | Tick proteins | H. truncatum, O. savignyi, | Cattle, sheep, others | Fever, sweating, anorexia, tearing, salivation; |
| other tick toxicosis) | O. lahorensis, A. persicus | high mortality | ||
hosts and are critically important for the expansion and spread of the tick populations, but they play no direct role in the perpetuation of the infection.
In the United States, LD has increased more than 1.7 times since it was first designated a reportable disease in 1991. By 1999, 16,273 cases that met the U.S. Centers for Disease Control and Prevention definition had been reported, for an overall incidence of 6.0 per 100,000. Most cases were reported from the northeastern, mid-Atlantic, and north central United States. Other important foci are in northern Wisconsin, northern Minnesota, and northern California. In Europe, important foci occur in the Scandinavian countries and in Germany, Poland, the Czech Republic, and Russia.
Domestic animals are also susceptible to infection with Borrelia pathogens. Dogs, cats, cattle, horses, and possibly other livestock and companion animals were found to be infected with B. burgdorferi. High seroprevalence rates occur in hyperendemic areas such as the northeastern United States, where many dogs show typical symptoms of chronic LD, especially lameness in one or more legs, fever, and fatigue.
Rocky Mountain Spotted Fever
RMSF occurs throughout almost the entire United States, southern Canada, and Mexico and, to a lesser extent, in South America. This disease is caused by a tiny intracellular bacterium, the rickett-sia Rickettsia rickettsii. People become ill with RMSF following the bite of an infected adult tick. Once in the human host, the rickettsia multiply profusely in the epithelial linings of the capillaries, arteri-oles, and venules. Vessels hemorrhage which in the dermis of the skin leads to the characteristic red spots. These innumerable reddish lesions, raised above the skin surface (maculopapular rash), coalesce to form the characteristic spotted rash. Patients also develop high fever, severe headaches, nausea, joint and muscle pain, photophobia, and other symptoms. Unless treated with antibiotics, some patients die and others suffer irreversible injury.
In the United States and southern Canada, the primary vectors of RMSF are the Rocky Mountain wood tick, Dermacentor andersoni, in the west and D. variabilis in the east. Adults of these tick species readily attack humans. Other tick species (e.g., the rabbit ticks Haemaphysalis leporispalustris and Ixodes dentatus) transmit the rickettsia when they feed on rabbits and birds, thereby contributing to the maintenance of the zoonosis in nature, but these latter species do not bite humans. The disease survives in the natural environment in overwintering (i.e., diapausing) ticks but not in the reservoir hosts. When rickettsia-infected larvae emerge from diapause in the spring, they infect mice and other susceptible rodents on which they feed. Other, uninfected larvae feeding on the rickettsemic animals acquire the infection, and the disease spreads rapidly in the tick population. Nymphal ticks spread the infection further as they feed again on other small mammals. Adult ticks seek larger animals (e.g., dogs and raccoons) as well as humans. Rickettsia are passed to subsequent generations of ticks by transovarial transmission. Ticks also harbor nonpathogenic species such as R. montana, R. belli, R. rhipi-cephali, R. parkeri, and other as yet unnamed rickettsia, complicating attempts to measure the incidence of RMSF in nature.
In the United States, about 600-800 cases of RMSF have been
reported yearly since 1985, with an estimated annual incidence between 0.24 and 0.32 per 100,000 population. Most cases now occur east of the Mississippi River, with the highest concentration in the south-central and southeastern states, especially along the Atlantic coast. Cases tend to occur in foci in rural areas and suburban communities near major population centers. Since RMSF is a seasonal disease, the frequency of cases follows the seasonal activity pattern of the adults, with highest frequency in July and August in the southern United States but greater frequency in May and June in the northeastern part of the country.
A closely related disease, boutonneuse fever (Mediterranean spotted fever), caused by R. conorii, occurs in southern Europe, North Africa, and Asia. These rickettsia are transmitted to humans by the bite of the brown dog tick, Rhipicephalus sanguineus. Although generally similar in its symptoms to RMSF, this illness is distinguished by the formation of a black ulcer, the eschar, at the wound site where the infected tick had attached.
Ehrlichiosis
Another rickettsial disease that has emerged into increasing prominence in recent years is ehrlichiosis. The causative agents are known as ehrlichiae, tiny obligate intracellular organisms that invade the white blood cells of humans and animals. Ehrlichiae develop within cytoplasmic vacuoles in different blood cell types such as monocytes, granulocytes, lymphocytes, or even platelets, depending on the species. Patients develop an acute illness with high fever and severe headaches, as well as aching muscles and joints. In contrast to RMSF, a rash is not common (20-30% of patients) and usually does not involve the palms or soles. Although severe cases can occur and may result in death, most cases are relatively mild. In the southeastern United States, most cases are caused by Ehrlichia chaffeen-sis, which invades the monocytic leukocytes and causes leukopenia (abnormal loss of white blood cells) among other symptoms. This disease is now known as human monocytic ehrlichiosis (HME). The primary vector for E. chaffeensis is the lone star tick, Amblyomma americanum. These ehrlichiae develop primarily in the monocytes. Some cases are caused by E. ewingii, also transmitted by lone star ticks. In the northern and western United States, the disease is caused by a related ehrlichia, E. phagocytophila (E. equi), which also causes illness in livestock. However, like E. ewingii, these ehrlichiae prefer the granulocytic leukocytes and, for this reason, the disease is known as human granulocytic ehrlichiosis (HGE). The primary vector in the northern United States is I. scapularis. In California, the primary vector for humans is I. pacificus. In Europe, the pathogen is transmitted to humans by the bites of the I. ricinus. E. phagocy-tophila, believed to be the most widespread of the various human-infecting ehrlichiae, has been reported in many countries of Europe and northern Asia. Ehrlichiosis also affects dogs, sheep, and other animals, being caused by different ehrlichiae.
Tick-Borne Encephalitis
Tick-borne encephalitis (TBE) is caused by viruses of the family Flaviviridae. In Europe and northern Asia, the illness in humans is manifested by high, often biphasic, fever and headache, followed soon afterward by inflammation of the brain (encephalitis) and meninges (meningitis). Some patients develop muscle weakness or paralysis, especially in the right shoulder muscles. In the Far East, case fatality rates are relatively high (up to 54%), whereas the disease in Europe is considerably milder. In Europe and the Far East, TBE viruses are transmitted by I. ricinus and by the taiga tick, I. persul-catus. Other tick species that feed on wild animals may amplify viral infection. Rodents and insectivores are the chief reservoir hosts. The disease is endemic in central, northern, and eastern Europe, where thousands of cases are reported each year. In India, a similar disease known as Kyasanur forest disease has been responsible for numerous cases of human illness. In North America, a Flavivirus of the TBE complex causes a disease known as Powassan encephalitis, a serious illness that has caused death in some patients and permanent nerve damage in others. The primary vector to humans in the east is a little-known tick, I. cookei, a common parasite of woodchucks, foxes, and raccoons; in the west, it is transmitted by the D. andersoni.
Babesiosis and Theileriosis
Two genera of tick-borne protozoa, Babesia and Theileria, cause severe illness and even death in domestic livestock and wildlife throughout most of the world. Tick-borne babesiosis can also cause illness in humans. B. bigemina, B. bovis, and B. divergens are examples of tick-borne protozoans that can cause babesiosis, a serious and often fatal illness in cattle prevalent in Mexico, parts of South America, Africa, and elsewhere. The clinical course of the disease is characterized by high fever and dehydration. Sick animals stop feeding, become lethargic, and show labored breathing. Anemia and bloody urine (so-called redwater fever) is a consequence of the massive destruction of red blood cells. Although some animals recover naturally, most of the diseased individuals gradually become comatose and die. Mortality estimates for infections with B. bigemina are 30%,with B. bovis are 70-80%. The causative agents, which are introduced by the bites of infected ticks, develop as meronts in the eryth-rocytes and multiply by multiple fission or, in the case of B. bigemina, by binary fission. Subsequently, huge numbers of erythrocytes are destroyed, leading to the symptoms noted. Eventually, some parasites invade leukocytes, where they are transformed into piroplasms, the stage that will infect ticks. Piroplasms remain in an arrested state of development until ingested by a suitable tick vector. In the tick’s digestive tract, the erythrocytes and leukocytes of the host blood are lysed, releasing the parasites. The meronts are destroyed, but the piroplasms develop into ray-shaped gamonts (strahlenkorpers) that fuse with other, similar gamonts to form the diploid zygotes. These parasites are transformed into cell-penetrating ookinetes that traverse the tick’s midgut epithelium and seek out the ovaries. In female Boophilus ticks, numerous oocytes are invaded by these ook-inetes. When the ticks mate and lay eggs, the ookinetes proliferate in the developing embryos. Eventually, as the embryos mature, the ookinetes move to the salivary glands of the young larvae, where they form the invasive stage known as sporozoites, ready to infect cattle. Thus, a single blood meal in a female tick can result in thousands of disease-infected larval progeny, a phenomenon known as transovarial transmission. As a result, numerous animals or even an entire herd of cattle can be infected in a single tick generation.
Human babesiosis is a malaria-like illness caused by B. microti, B. gibsoni, B. major) or B. divergens. In the northeastern and mid-western United States, the dominant pathogen afflicting humans is B. microti, transmitted by the bite of I. scapularis. In Europe, human babesiosis may be caused by B. microti, or B. divergens, transmitted by I. ricinus and/or other ixodids. Although the life cycle in the human body is similar to that of the bovine babesias, development in the tick is quite different. When blood infected with B. microti is ingested by immature deer ticks, the invasive stages of the parasite transform into cell-penetrating sporokinetes, which, aided by a cell-piercing arrowhead organelle, traverse the midgut epithelium and find their way to the salivary glands. When the fed ticks molt to the next feeding stage and attack new human or animal hosts, the sporo-zoites are injected with the tick’s saliva.
Babesiosis also afflicts other animals. In dogs, B. canis, transmitted by the bites of the R. sanguineus, causes severe illness and may be fatal. Other babesias cause illness in horses, sheep, and other animals.
Protozoan parasites closely related to the babesias, Theileria parva and T. annulata, cause serious or fatal illness in cattle and other ungulates. T. parva, transmitted by the bites of the African brown ear tick,Rhipicephalus appendiculatus, causes a disease in southern Africa known as East Coast fever. T. annulata, transmitted by the bites of ticks of the genus Hyalomma, causes a disease known as tropical theileriosis. It is much more widespread, ranging from North Africa across western and central Asia to the Indian subcontinent. The life cycle of both protozoan parasites is generally similar to that of the babesias but with several notable exceptions. Theileria parasites multiply in the leukocytes instead of the erythrocytes. Following destruction of the host cells, the Theileria progeny infect other leukocytes, thereby damaging the host’s immune system, while others invade erythrocytes to form piroplasms. The latter are the stage that will infect ticks. These piroplasms remain in an arrested state of development until ingested by the tick vector. When vector-competent ticks feed on Theileria- infected cattle, the piroplasms are released from the disintegrating erythrocytes and transform into gamonts similar to those seen in the babesias. Following gamont fusion and fertilization, the zygotes develop into motile kinetes that cross the midgut epithelium and travel to the salivary glands. Because only the salivary glands are infected, pathogen transmission occurs only when the infected larval or nymphal ticks molt and feed again in the next life stage on susceptible hosts (transstadial transmission).
Tick Paralysis and Tick Bite Allergies
In addition to transmission of infectious microbes, ticks may cause paralysis, allergies, and severe toxic reactions in their hosts. In humans, this affliction is manifested by a gradually ascending paralysis, beginning with the loss of sensation and motor coordination in the legs, abdominal muscles, back muscles, and, eventually, the diaphragm and intercostal muscles. Unless the tick responsible for these symptoms is found and removed, the patient will undergo respiratory failure and die. Most patients recover rapidly after tick removal, but those with advanced symptoms may require several weeks for complete recovery.
Tick paralysis also affects animals, especially large ungulates. In Australia, a single Ixodes holocyclus female may be sufficient to cause the death of a full-grown cow or bull. Thousands of cattle died in the western United States and Canada because of paralysis induced by the bites of D. andersoni ) Other than finding and removing all the feeding female ticks, there is no known cure. Some ticks also cause severe allergic and toxic reactions. A notable example is the African tampan Ornithodoros savignyi, the bite of which may cause cattle to salivate, tremble, gnash their teeth, become disoriented, and even die. A similar condition, known as sweating sickness, is caused by ticks of the genus Hyalomma, especially H. truncatum. The illness is characterized by fever, loss of appetite, sweating, lachrymation, salivation, and, usually, death. However, there is no paralysis.
CONTROL
Although difficult to estimate in precise monetary figures, the economic importance of ticks is enormous. This is primarily because of losses caused by tick-transmitted diseases affecting livestock. In addition, ticks cause injury from severe blood loss, reduced milk production, and damage to the hides. Worldwide, losses to the cattle industry alone were estimated to be more than $7 billion. Perhaps the most important economically are the cattle ticks, especially Boophilus annulatus, B. decoloratus, and B. microplus, because of the disease agents they transmit (Babesia spp.). Other economically important species are the bont ticks, Amblyomma variegatum and A. hebraeum, which are vectors of the causative agent of heartwater, Cowdria ruminantium.
Tick control is done almost exclusively with acaricides: poisonous compounds such as chlorinated hydrocarbons (e.g., DDT), orga-nophosphorus compounds (e.g., diazinon), carbamates (e.g., carbaryl), and pyrethroids (e.g., permethrin and flumethrin). Animals are treated with acaricides by either bathing or spraying them with mixtures of liquids containing the specific toxicant. Acaricides can also be delivered as pour-ons or spot-ons, highly concentrated mixtures containing surfactants (i.e., spreading agents) that enable the product to disperse naturally throughout the animal’s hair coat or as dusts, consisting of mixtures of talc and the acaricide. For long-lasting effects, acari-cides are incorporated into plastic collars, ear tags, or even tail tags, which gradually emit the toxic chemicals over a long period of time. Also promising is the tick decoy in which tail tags are impregnated with the tick’s natural pheromone as well as the acaricide. These “tick decoys” demonstrated excellent efficacy (>95%) for up to 3 months when applied to cattle in Zimbabwe. Other promising innovations for treating tick-infested animals include self-medicating devices, such as the “four-poster” developed by the U.S. Department of Agriculture for treating white-tailed deer, and antitick vaccines. Self-medicating applicators release an acaricide from a reservoir when host animals insert their heads into the device to retrieve corn or other bait. This is perhaps the first practical method that has been developed for controlling ticks on wild animals without killing or injuring them. Antitick vaccines work by immunizing livestock animals such as beef cattle against proteins in the tick’s digestive tract, thereby killing the ticks as they attempt to feed. Further scientific advances may make it possible to reduce the current dependence on toxic chemicals to control ticks on domestic animals, pets, and even wildlife.
Hikers, campers, and other people entering tick-infested habitats should take appropriate precautions to avoid ticks and the risk of acquiring tick-borne diseases. Each person should wear long trousers, tuck the trouser ends into boots or shoes, and draw the socks over the trousers. The protective barrier should be secured with tape and treated with a repellent—for example, one of the many products that contain diethyl toluamide (DEET). Upon returning from the trip, each person should decontaminate the field clothes and carefully inspect skin and hair for ticks that may have attached unnoticed. Early detection and removal of attached ticks will minimize the risk of transmission of disease-causing agents. Unattached ticks, if any, may be destroyed and discarded. However, attached ticks should be removed carefully with tweezers (forceps) and retained intact if needed for future examination in case of illness.
