The Sternorrhyncha, comprising some 16,000 described species, is one of the suborders of the order Hemiptera (the true bugs). It contains four major groups, all entirely phytophagous, and usually recognized as superfamilies: the Psylloidea (psylloids or jumping plant-lice); Aleyrodoidea (whiteflies); Aphidoidea (aphids or aphidoids); and Coccoidea (scale insects or coccoids). The name “Sternorrhyncha” (from the Greek “sternon” meaning chest and “rhynchos” meaning nose or snout) refers to the location of the mouthparts on the underside of the insect, between the bases of the front legs, although sometimes the mouthparts are lacking in the adult.
OVERVIEW OF STERNORRHYNCHA
Insects belonging to the Hemiptera are unique in having their mouthparts forming a rostrum that comprises mandibles and maxillae modified as needle- or thread-like stylets lying within a grooved labium. Two pairs of stylets interlock to form two canals, one delivering saliva and the other uptaking plant or animal fluid. The Sternorrhyncha is widely accepted as the sister group to the rest of the Hemiptera. It is a well-defined group diagnosed by the position of the rostrum and the presence of only one or two segments on each tarsus (the part of the leg most distant from the body and bearing one or two claws); in most other hemipterans there are three tarsal segments.
Sternorrhynchans use their stylets to probe plant tissues intracel-lularly (into or through plant cells) or intercellularly (between plant cells). The tips of the stylets always enter cells at the site of ingestion, which is often phloem-sieve elements. Generally, stylet penetration is accompanied by secretion of a solidifying saliva that forms a sheath around the stylets. Other hemipterans mostly probe intracellularly, may or may not secrete salivary sheaths, and ingest from a wider variety of plant or animal tissues. Most sternorrhynchans are phloem feeders, and thus have a diet rich in carbohydrates (sugars) and deficient in amino acids and other nitrogenous compounds. Generally, there is an intimate association with intracellular bacteria, called endosymbionts, which are housed in special tissue (bacteriomes or mycetomes) and contribute nutrition to the insect host.
Sternorrhynchan excreta is a sticky, sugary liquid called honeydew that may contaminate foliage; it serves as a substrate for the growth of black sooty mold fungi that can impede photosynthesis and reduce plant vigor. Honeydew often attracts ants that may protect the ster-norrhynchans from their natural enemies, especially predatory and parasitic insects.
Adult jumping plant-lice, adult whiteflies, and many adult aphids have two pairs of wings. All adult female scale insects and most adult female aphids are wingless (apterous). Adult male scale insects usually resemble small flies (order Diptera) in having the hind wings reduced to small balancers (halteres), although sometimes the hal-teres are absent; in a few scale insect species males are apterous. The absence of wings in adult females of scale insects and many aphids means that the immature stages (nymphs) of these groups can be difficult to distinguish from their adults.
Evolutionary interpretations of morphology have suggested either that whiteflies are sister to aphids + scale insects with psylloids sister to all three, or that whiteflies + psylloids are sister to aphids + scale insects. Phylogenetic analysis of nucleotide sequences (of small subu-nit rDNA, also called 18S rDNA) supports the former hypothesis that Psylloidea is the sister group to the other sternorrhynchans. Some of the morphological traits shared by Aleyrodoidea and Psylloidea are ancestral features (plesiomorphies) such as two, similar-sized, tarsal segments (the first tarsal segment is reduced or absent in aphids and coccoids) and good jumping ability. Also both groups have pedunculate eggs, that is, one end of the egg has a short stalk or narrow extension (Fig. 1 – . Whiteflies, aphids, and scale insects all have Malpighian tubules (filamentous excretory organs) reduced in number or absent and their gut often has a well-developed filter chamber that allows most of the water in ingested sap to bypass the absorptive part of the midgut. In contrast, psylloids have four Malpighian tubules (an ancestral feature found in other Hemiptera) and possess only a rudimentary filter chamber.

FIGURE 1 The life cycle of Cardiaspina fiscella (Psylloidea: Psyllidae).
PSYLLOIDEA (JUMPING PLANT-LICE)
Worldwide, there are only about 2500 species of psylloids or jumping plant-lice, often collectively called psyllids despite there being 6 included families. The greatest abundance and species richness of psylloids are found in tropical and south temperate regions, where many new species await discovery. In the Northern Hemisphere, however, fewer than 100 species occur in Britain and only about 300 species are known from North America. Adult psylloids are small, ranging in length from 2 to 8 mm, and superficially resemble miniature cicadas or small leafhoppers, but are distinguished by their multi-segmented antennae. Their common name derives from the ability of adults to jump backwards when disturbed. All psylloids feed by sucking sap, mostly of woody dicotyledonous plants. They ingest sap from a variety of tissue sources, including phloem, xylem, and mesophyll parenchyma, and thus are not phloem-specialists like most other sternorrhynchans. Psylloid nymphs are either free-living, gall-inducing, or lerp-forming (Figs. 2-4); lerps are manufactured sugary and starchy covers or scales under which the nymphs live. Some species of jumping plant-lice are pests of cultivated plants, with damage resulting from loss of plant sap, toxins in the saliva, and/or disease caused by transmitted microorganisms.

FIGURE 2 Adult (right) and nymph of Paurocephala bifasciata (Psyllidae) on Ficus hispida, Hong Kong.

FIGURE 3 Galls of Schedotrioza species (Triozidae) on a Eucalyptus leaf, Victoria, Australia; two galls have split open at the apex, allowing the adults to escape.
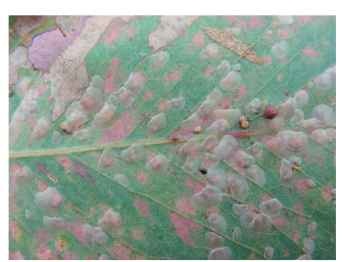
FIGURE 4 Lerps of Cardiaspina albitextura (Psyllidae) on leaf of Eucalyptus blakelyi, Canberra, Australia; nymphal feeding has caused leaf necrosis and a late-instar nymph with its lerp removed is visible.
Evolution and Classification
Historically, the classification of psylloids was based on the well known, but relatively species-poor, Holarctic fauna. Expanding knowledge of the diverse tropical and south temperate faunas has led to better understanding of relationships and hence a more natural classification. The system used here is based on that of White and Hodkinson, presented in 1985, with emendations suggested by D. Burckhardt and D. Hollis. In this system, 6 families of extant psylloid species are recognized (with each of the first 4 families listed here having 10 or fewer genera): Calophyidae, Carsidaridae, Homotomidae, Phacopteronidae, Psyllidae (includes the Aphalaridae, Liviidae, and Spondyliaspididae, recognized by some authors; about 120 genera), and Triozidae (over 40 genera).
Many species of the predominantly Neotropical and tropical/subtropical Asian family Calophyidae feed on plants in the Anacardiaceae. Carsidaridae is almost entirely pantropical in distribution and is restricted to the related plant families Bombacaceae, Malvaceae, and Sterculiaceae. The Homotomidae is predominantly from the Old World tropics and feeds almost exclusively on figs (Moraceae: Ficus). The Phacopteronidae occurs in tropical Asia, Africa, and the Americas and many species feed on plants in the Meliaceae. The Psyllidae is the largest family, but it is heterogeneous morphologically. A large number of Psyllidae are associated with legumes (Fabaceae), although the speciose subfamily Spondyliaspidinae specializes on Eucalyptus (Myrtaceae). Many species of Spondyliaspidinae produce lerps or induce leaf galls that they plug with lerp substance. The Triozidae is cosmopolitan and has wide-ranging host preferences.
The fossil record of at least stem group Psylloidea extends back into the Permian (>270mya) and the extant families probably diversified in the Cretaceous. Relationships among psylloid higher groups (families and subfamilies) are largely unresolved.
Life History
Reproduction is always sexual. There are seven life stages (Fig. 1): the egg, which is stalked; five nymphal instars; and the adult, with the males and females in about equal numbers. Eggs are either completely inserted into plant tissue or inserted by just the stalk (peduncle) through which water is absorbed. Depending on species, each female may lay as few as 20 to more than a thousand eggs. In cold temperate regions, psylloids frequently have only a single generation per year (univoltine). In warmer climates, development continues year round, often with several to many generations per year. Species with multiple generations per year (polyvoltine) are more likely to have population outbreaks than univoltine or bivoltine species.
Morphology
Identification is based primarily on adult characteristics, with males being most important for species-level determinations, although features of the female terminalia are sometimes used. The fifth-instar nymph can materially aid identification in many groups. Adult males and females are morphologically similar, except that males are often smaller, have different genitalia, and thus different abdominal shapes. In a few species the sexes are color dimorphic, and there is often a seasonal color dimorphism. Body coloration or the pattern of markings can be diagnostic of species. The size and shape of the head and its appendages provide useful taxonomic characters. Immature psylloids are usually characteristically flattened dorsoventrally and many secrete wax.
Behavior and Ecology
Adult psylloids disperse over short distances by jumping or flying, but many species may travel long distances on air currents. The adults of a number of species are known to stridulate as a method of mate attraction. Mating behavior is often rudimentary, but precopu-latory darting movements and wing flicking have been observed in some species of Spondyliaspidinae.
The habits of nymphs vary among the different taxonomic groups. Nymphs of many species are naked (Fig. 2), although often covered in waxy body secretions, and live exposed on the shoots of their host plant. Others induce galls of various complexity, from simple pits in leaves to round or elongate structures that totally enclose the nymphs (Fig. 3). A third lifestyle is for the nymphs to develop on foliage but protected under scale-like covers called lerps (Fig. 4- . Each lerp is formed from substances eliminated from the insect’s gut (a special kind of liquid feces that is different from honeydew), or of a mixture of “feces” intermingled with wax filaments (exuded from pores in the anal plate). The “feces” of at least some species are composed of starch, especially amylose and amylo-pectin, synthesized in the insect’s gut. The insect builds the lerp by moving its abdomen back and forth while squeezing out the liquid excreta (like glue from a tube), which rapidly hardens to form a lacework of filaments. Lerps have various shapes and sizes, from simple cones to fans or lacy shell-like structures, and often are of diagnostic use. Lerps vary in color from white to cream or brown, and may be opaque or transparent. Some lerp-formers also (e.g., Glycaspis spp.) produce copious honeydew, whereas others (e.g., Cardiaspina spp.) produce very little or none.
Most species are specialist feeders as nymphs, with many species restricted to one plant genus or even a single species, and often to particular host structures (leaves, new shoots, etc.) or growth stages (either young or mature foliage). Most psylloids develop on dicots, a few on monocots or conifers, but none on ferns. In cool-temperate climates, some species overwinter as adults on evergreens, returning to their true hosts in spring, when eggs are laid. Host selection appears to involve taste rather than olfaction. Adults frequently are less discriminating than nymphs and thus host plants are defined as those on which nymphs can complete their development to adulthood. Nymphal feeding frequently damages the host plant (e.g., premature leaf senescence), whereas adult feeding usually causes little injury. Many psylloid species have a mutualistic association with honeydew-seeking ants.
Notable Pest Species and Their Control
Some species of jumping plant-lice have become pests of plantation trees (e.g., Ctenarytaina spp., Glycaspis brimblecombei, Eucalyptolyma maideni and Blastopsylla occidentalis on Eucalyptus spp., all now established in California).
Two of the world’s most serious citrus pests are the Asian citrus psylla, Diaphorina citri (Psyllidae), and the African citrus psylla, Trioza erytreae (Triozidae). Other than the citrus pests, the most economically important psylloids of temperate and subtropical areas feed on cultivated apple and pear trees (e.g., Cacopsylla mali, C. pyricola, and related species of Psyllidae). Members of the Triozidae occur as pests on persimmons, cultivated fig, carrots, potatoes, tomatoes, and other Solanaceae. The leucaena louse, Heteropsylla cubana (Psyllidae), a Caribbean native, has become a pest of the tropical leguminous tree Leucaena leucocephala in several parts of the world where it is planted for a range of purposes.
Recorded parasitoids are mostly psylloid specialists, but there is little evidence of parasitoid-host specificity. Worldwide a number of wasp species, especially from the families Encrytidae (e.g., Psyllaephagus spp.) and Eulophidae (e.g., Tamarixia spp.), are parasitic on psylloids. In Europe, cecidomyiid flies (Endopsylla) are parasitic on some adult psylloid pests (e.g., Cacopsylla spp.).
ALEYRODOIDEA (WHITEFLIES)
Members of the Aleyrodoidea derive their common name from the powdery, white waxy secretion preened over the body and wings of most adults. The Greek root “aleuro,” found in many whitefly names, means “flour.” Adults ofboth sexes are tiny, delicate, and free flying. They have a wingspan of up to 11 mm, but usually about 2 mm, and resemble minute moths. They are a familiar sight to many home gardeners because, when infestations are heavy, the adults will fly up enmasse if disturbed from the foliage of favored host plants. The group occurs worldwide although few whiteflies occur in the cooler temperate regions. About 1560 species have been described, with perhaps two or three times as many species awaiting collection and formal taxonomic study. All adults and nymphs feed by ingesting phloem sap and several species are serious plant pests.
Evolution and Classification
All whitefly species belong to one family, Aleyrodidae, which is divided into just two accepted subfamilies, the Aleurodicinae and Aleyrodinae. The Aleurodicinae contains 20 genera and 130 described species, mostly from Central and South America, and the Caribbean. All other species (in around 140 genera) belong to the Aleyrodinae, which has a mostly pantropical and warm-temperate distribution. The two subfamilies are defined on both adult and nymphal features, whereas species and genera are diagnosed mainly or entirely on characters of the fourth-stage nymph, usually known as a puparium. The Aleurodicinae generally have larger adults (the largest is a centimeter long) with more complex wing venation than those of the Aleyrodinae.
Whiteflies are rarely fossilized because they have small and delicate bodies. The known fossils are preserved mostly in Cretaceous and Tertiary amber with one record from the Upper Jurassic. The two modern subfamilies probably diverged during the Cretaceous.
Life History
Reproduction is usually sexual with fertilized eggs producing females and unfertilized eggs producing males (arrhenotoky or arrhenotokous parthenogenesis); unmated females lay only haploid eggs. A few species produce only females (thelotoky or thelotokous parthenogenesis). There are six life stages (Fig. 5): the egg stage; the first instar, in which the nymph is often called a crawler; the second and third instars, in which
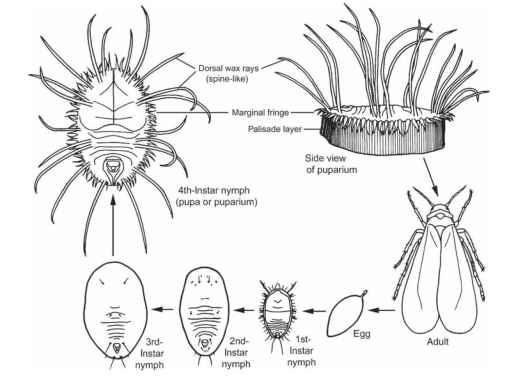
FIGURE 5 Life cycle of the greenhouse whitefly Trialeurodes vaporariorum (Aleyrodoidea: Aleyrodidae).
the nymphs are sessile; the fourth instar or last nymphal stage at the end of which the insect is usually referred to as a “pupa,” or a “puparium,” although it is not a true pupa as seen in holometabolous insects as there is no molt to a totally non-feeding, “resting” stage; and, finally, the adult (or imaginal) stage (Figs. 6 and 7). After the emergence of the adult, the molted cuticle (exuviae) is often called a “pupal case.”
Whitefly eggs are usually attached to the underside of leaves by a short stalk, the pedicel, through which water is absorbed from the plant. Eggs are often laid in circles or arcs (Figs. 6 and 7 ), and egg batches are frequently conspicuous because of a dusting of white wax. First-instar nymphs usually settle adjacent to the eggs from which they hatch. In most species, development occurs on the underside of leaves. After the first molt, the nymphs become immobile and cannot move to a new site if food quality deteriorates. During the fourth instar, feeding ceases and the insect transforms into the winged adult.

FIGURE 6 Adults and egg spirals of Aleurodicus pulvinatus (Aleyrodidae: Aleurodicinae) on leaf of Coccoloba uvifera, Aruba.

FIGURE 7 Adults and egg circles of Aleurothrixus floccosus (Aleyrodidae: Aleyrodinae) on leaf of cultivated Plumeria species, Ecuador.
Species such as the greenhouse whitefly (Trialeurodes vaporari-orum) can breed continuously in greenhouses or indoors. Those whitefly species that have economic impact have several to many generations per year, but many other species have only one or two generations annually.
Morphology
Historically, puparial rather than adult characteristics have been used to recognize species and genera, although adults do display many taxonomically useful characteristics. Seasonal dimorphism, as seen in many aphid species, is uncommon but occurs in the puparia of a few temperate species. Male and female whiteflies are similar in appearance except for their genitalia, but males are often smaller than females of their own species. The body and wings are dusted with powdery wax emanating from large, paired wax plates on the underside of the abdomen and thus adults most commonly appear whitish or grayish even if the body color is yellow, brown, red, or if they are darkly sclerotized. The male genitalia (the claspers or para-meres and the intromittent organ or aedeagus) are more informative taxonomically than the female terminalia (ovipositor and associated structures), especially in the Aleurodicinae.
Nymphs of all stages (Fig. 5) superficially resemble scale insects (Coccoidea) and frequently have ornate wax secretions in later instars. The anus of all life stages opens in a special dorsal pit, near the posterior end of the abdomen, called a vasiform orifice which comprises a depression, a dorsal flap (the operculum), and a tongue-shaped lingula that is used to flick away each droplet of anal excreta (honeydew) before it can accumulate. The lingula is the only mobile body part in instars II-IV
Puparia are mostly oval or elongate-oval, 0.5-2mm long, their color varying from transparent to white, brown, or black (Fig. 8 ). Waxes contribute to puparial protection and may be invisible or highly ornate, white or grayish, but mechanical color (iridescence) may be beautiful blue, turquoise, bottle-green, or pearly-white. Visible wax may form dorsally and/or as a marginal fringe.
Behavior and Ecology
Adult males of a few whitefly species, especially Bemisia tabaci and T. vaporariorum, have been observed to display courtship behavior prior to mating, including abdominal oscillations that result in acoustic signals caused by substrate-borne vibrations. Females may produce sex pheromones to attract males. Adult whiteflies will fly short distances if disturbed from their host plant. They also undertake
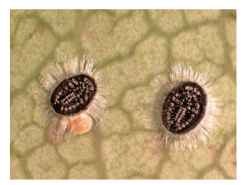
FIGURE 8 Puparia of Aleuroparadoxus arctostaphyli (Aleyrodidae: Aleyrodinae) on leaf of Arbutus menziesii, California, USA.
longer migratory flights, which are air-current dependent because whiteflies are weak fliers. They have complex host-finding and host-orientation behaviors, at least involving attraction to particular colors, especially yellow or yellow-green.
Whiteflies feed from plant vascular tissue and all feeding stages produce copious quantities of honeydew, yet few species are ant-attended. Most whitefly species appear to be oligophagous, with few known to be monophagous; however, polyphagous species are the most documented because they are most likely to be pests. Whitefly host plants are almost entirely flowering plants (angiosperms), especially woody dicots; relatively few species of whiteflies are found on herbs, grasses, ferns, or palms, upon which plant groups they do appear to specialize.
The morphology of the puparia of some species has been shown to vary depending on the species of plant that is acting as host, probably because of differences in the nature of the plant surfaces. For example, in B. tabaci the size, number, and position of setae of the puparia can be correlated with leaf hairiness. These phenotypes of the same species often look very different from each other and this phenomenon can confound identification. This variation is especially a problem among polyphagous species in the genera Bemisia and Trialeurodes.
Notable Pest Species and Their Control
The feeding activity of many whiteflies damages their host plants and some species are known or believed to transmit diseases, especially those caused by viruses. The most serious aleyrodid pests are those of orchards (especially citrus), greenhouse crops, and some ornamentals such as orchids and aroids, and a few pest species are almost cosmopolitan in distribution. The past two decades have seen an increase in the pest status of certain whiteflies, probably because of the development of aggressive strains that are more fecund, more efficient virus vectors, and/or have broader host ranges. Successful whitefly control depends upon an appropriate integrated pest management program (IPM) in which natural enemies are protected or augmented. A number of groups of parasitic wasps (particularly Eulophidae and Aphelinidae) specialize on whiteflies and useful predators include some ladybird beetles (Coccinellidae). Whiteflies also are attacked by pathogenic fungi, especially Aschersonia species.
One of the most important pests is the polyphagous tobacco or sweet potato whitefly, B. tabaci, which occurs predominantly in tropical and subtropical regions and on protected crops elsewhere. It feeds on numerous fiber, food, and ornamental plants, and damage is exacerbated by its ability to vector more than 70 different viruses. B. tabaci exists in many biotypes or strains, and one virulent form is known as biotype B or the silverleaf whitefly.
Other important whitefly pests include the cosmopolitan and polyphagous greenhouse whitefly, T. vaporariorum, the spiralling and giant whiteflies (Aleurodicus dispersus and A. dugesii, both Neotropical natives), Siphoninus phillyreae (perhaps of Middle Eastern origin), Parabemisia myricae (originally from Japan), the Indian grass-feeding species Vasdavidius (formerly Aleurocybotus) indicus, the citrus woolly whitefly (Aleurothrixus floccosus, endemic to the Americas but now established in Africa and south-east Asia), and Dialeurodes citri and Singhiella (formerly Dialeurodes) citrifolii (widespread pests of citrus).
APHIDOIDEA (APHIDS)
Aphids are small soft-bodied insects, ranging from 1 to 8 mm in length. They are usually found living in aggregations on rapidly growing parts of their host plants and their center of diversity is the northern temperate regions of the world. More than 4300 species are known. The life cycles of aphids are frequently complex and usually include parthenogenetic (or asexual), but often also sexual, reproduction; many species display host alternation in which cyclical parthenogenesis is combined with the obligate use of two unrelated host plants. Also many aphids produce either eggs or living young at different parts of the cycle. Adults may be winged (alate) or wingless (apterous). Most aphids can increase their population size rapidly because of parthenogenetic viviparity combined with “generational telescoping,” whereby a mother aphid carries both her daughters and their daughters (i.e., embryos within embryos). Aphid feeding activities can have deleterious effects on their host plants mainly via sap removal and/or virus transmission.
Evolution and Classification
The Aphidoidea contains three families: Phylloxeridae, Adelgidae, and Aphididae. Strictly speaking, the common name of the entire group should be ” aphidoids, ” but “aphids” is used almost universally as a collective name. Very occasionally the name “aphids” has been applied to just members of the Aphididae, which should more properly be referred to as ” aphidids. ”
The Phylloxeridae contains about 75 species, in eight genera, divided between two tribes. The Phylloxerini has seven genera, feeding mostly on oaks (Fagaceae: Quercus), or hickory and pecans (Juglandaceae: Carya) , but with one pest species on grape vines (Vitaceae: Vitis) . The Phylloxerinini has a single genus associated with the willow family (Salicaceae). The Phylloxeridae is probably the oldest extant family, although only one fossil (Palaeophylloxera from the Lower Miocene) is known. Many phylloxerid species induce galls on their host plants; only a few species are host alternating.
Conifer woolly aphids, the Adelgidae, comprise 2 genera and about 50 species and are entirely Holarctic in distribution. Adelgids induce galls on their primary host, spruce (Picea), and move to other conifers as alternate hosts.
The largest family, Aphididae, has been divided into a number of subfamilies and tribes, but different authors frequently use different classifications. The 10 subfamilies listed here are those recognized in the monographs by Blackman and Eastop: Anoeciinae (34 species), Aphidinae (over 2700 species), Calaphidinae (including the Drepanosiphinae, Thelaxinae, and several other groups treated as subfamilies by some other authors, 400 species), Chaitophorinae (164 species), Eriostomatinae (formerly Pemphiginae, 319 species), Greenideinae (151 species), Hormaphidinae (176 species), Lachninae (355 species), Mindarinae (5 species), and Phloeomyzinae (1 species).
The oldest aphid fossils are from the Triassic (at 220-210 mya) but aphids may have originated in the Permian. Phylogenetic analysis of molecular data suggests that aphids underwent a rapid radiation into the current tribes after transferring from gymnosperms to angiosperms some time during the Upper Cretaceous. Furthermore, the ancestral aphid probably had a simple life cycle with host alternation evolving independently in each of the families and perhaps several times within the Aphididae.
Life History
There are six stages in the life history of an individual aphid: the egg or embryonic stage, four nymphal instars, and the adult. Aphids have evolved a range of annual or biennial life cycles and other adaptive strategies that often vary within as well as among species. This complexity can complicate the study of aphid biology. Furthermore, aphid-ologists have developed a special nomenclature for the different life stages and types. Life cycles are called holocyclic (Fig. 9) if a sexually
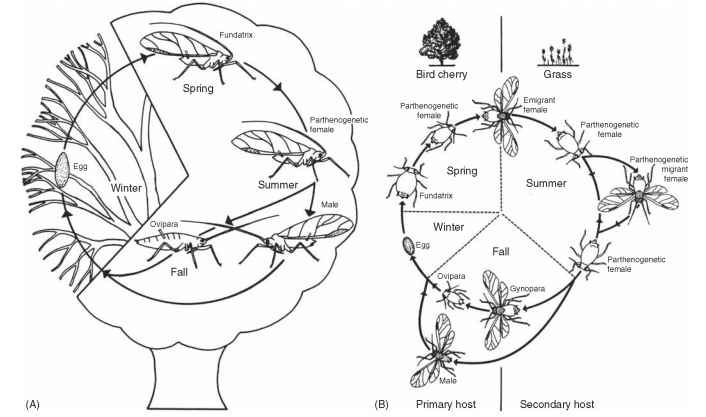
FIGURE 9 Life cycles of aphids: (A) The holocyclic life cycle of the monoecious sycamore aphid, Drepanosiphum platanoidis. (B) The holocyclic life cycle of the heteroecious bird cherry-oat aphid, Rhopalosiphum padi (fundatrix, foundress, a wingless parthenogenetic female that produces live offspring rather than eggs; gynopara, migrant that flies back to the primary host where she produces oviparae; ovipara, wingless, sexual egg-laying female that mates with a male).
reproducing generation is present, or anholocyclic if the sexual generation is absent. A typical complete cycle (holocycle) consists of a single generation of sexual morphs (sexuales) and several to many generations of only parthenogenetic females. In the sexual generation, males mate with females to produce fertilized eggs that are all female. Each egg gives rise to a wingless “foundress” or “stem mother” (fundatrix) that gives rise to a lineage of parthenogenetic females. The parthenogenetic descendants of a single foundress are called collectively a clone and are identical genetically but may be of different phenotypes (morphs). The adults may be wingless (apterae) or winged (alatae), depending on environmental conditions. In Aphididae, parthenogenetic females (vivi-parae) give birth to live young, whereas the sexual females (oviparae) lay eggs. In Adelgidae and Phylloxeridae, females lay eggs during both the sexual and asexual phases of reproduction. The phenomenon of cyclical parthenogenesis is a key feature of aphid biology. Some aphids have lost, or can facultatively lose, the sexual part of the life cycle (a condition called anholocycly). It is common for aphids to have 15-20 generations per year, with up to 40 in tropical climates.
Most aphids are monoecious (Fig. 9A) , that is, they undergo all phases of their life cycle on one host plant or on a small number of closely related hosts. About 10% of aphids are heteroecious (Fig. 9B), having more complex life cycles involving host alternation (heteroecy). The sexual morphs mate and the oviparae lay eggs on the primary host (often a deciduous woody plant); however, a regular migration occurs to another, unrelated plant, the secondary host (which may be either herbaceous or woody), on which the parthenogenetic generations live. The aphids must return to their primary host for the next sexual generation.
Host alternation may occur as part of a 1-year (annual) life cycle, as in many Aphididae, or as a 2-year (biennial) life cycle, as in Adelgidae. In the Aphididae, in temperate climes, alternation is frequently obligate, overcoming the twin problems of poor sap flow in woody primary hosts in summer, and the death of many herbs in winter.
Morphology
Aphids are highly polymorphic, with most species occurring in several different forms, or morphs. In individuals destined to be winged as adults, the wing buds are usually apparent after the second nymphal molt. Most common aphid species are soft-bodied and green in color, but dark and brightly colored species and a few hard-bodied species also occur. Nymphs superficially resemble apterous adults except that they are smaller in size and never have wings. Aphids usually have two prominent, tube-like structures on the posterior dorsum of the abdomen, called siphunculi or cornicles (Fig. 10 ), which can discharge defensive lipids and alarm pheromones. In some species, these structures are reduced to pores, or are absent. Another structure unique to the aphids is a posterior projection on the tip of the abdomen called the cauda, equivalent to the lingula in whiteflies. Protective wax secretions are common.
Behavior and Ecology
Both adults and nymphs can disperse short distances by walking, but migration of alatae occurs by both active flight and passive longdistance movement on air currents. Apterae and nymphs frequently

FIGURE 10 Apterae of Macrosiphum rosae (Aphididae) on Rosa sp., California, USA; paired siphunculi (=cornicles) visible at the end of the abdomen.
are transported by attendant ants. In sexual morphs, males are attracted to females by sex pheromones released from glands on the female’s legs. Altruistic behavior has been reported in the few aphid species that have a sterile soldier “caste,” which is a special kind of first- or second-instar nymph that defends the family group against competitors and natural enemies.
Aphids generally live in aggregations (Fig. 11) on the buds, stems, and/or leaves of their host plants. Some species induce galls and a few others live underground on roots. Almost all aphids are phloem feeders, producing copious quantities of honeydew, and thus often are attended by ants. Some aphidids feed from both phloem and parenchyma tissue, and adelgids are largely parenchyma feeders. In general, monoecious aphids are quite host specific, with each genus being associated with a particular host-plant family and each species with one genus or species of host plant or at least closely related plant genera. In host-alternating species, however, the primary and secondary hosts are usually unrelated and the specificity to the primary host is generally higher than to the secondary host(s). Pest aphids, especially those of agricultural crops, often feed on plants in a number of unrelated families.
Notable Pest Species and Their Control
Infestations of aphids can grow rapidly to enormous size and cause plant debilitation through nutrient deprivation, but even

FIGURE 11 An aggregation of Brevicoryne brassicae (Aphididae) on cabbage flower shoot, England.
small numbers of aphids can transmit viruses to uninfected plants. Pest aphids often are more polyphagous than non-pest species and frequently are species exotic to the area where they are of most economic concern. Cosmopolitan pest species are frequently anholo-cyclic, that is, reproducing continuously by parthenogenesis on their crop hosts, and thus are particular pests in the tropics.
One of the most notorious aphidoid pests is the grape phylloxera, Daktulosphaira vitifoliae (Phylloxeridae), which induces galls on Vitis species, including the viticulturally important V vinifera . The main method of control is the use of resistant grape rootstocks.
Genera of Aphididae that contain significant pests of crops include Aphis, Brachycaudus, Brevicoryne (Fig. 11), Dysaphis, Macrosiphum (Fig. 10), Myzus, and Rhopalosiphum. The species listed below all transmit plant viruses. There are a number of serious Aphis pest species, including A. craccivora, A. fabae, and A. gossypii. Other pests include B. brassicae (Brassicaceae), Ma. euphorbiae, My. ascalonicus, My. cerasi, My. persicae (whose secondary hosts include a range of economically important plants), R. maidis (a worldwide pest of cereal crops), Pineus species (Adelgidae, on pines), Cerataphis frans-seni (Hormaphidinae, on coconut and other palms), Cinara species (Lachninae, on conifers), and Eriosoma lanigerum (Eriosomatinae, on apple branches and trunks).
The most common aphidophagous predators are ladybird beetles (Coccinellidae), lacewings (Neuroptera), some hoverflies (Syrphidae), a few gall midges (Cecidomyiidae, especially the widespread Aphidoletes aphidimyza), and certain predatory bugs (Anthocoridae). Wasps of the large family Aphidiidae and several genera of Aphelinidae are endo-phagous parasitoids of Aphididae; Adelgidae and Phylloxeridae are not parasitized.
COCCOIDEA (SCALE INSECTS)
The scale insects (also called coccoids) occur worldwide. They are mostly small (less than 5 mm long) and often cryptic in habit although others are highly visible (Fig. 12). There are estimated to be almost 8000 species. Many scale insects are economically important pests of agriculture, horticulture, and forestry. Male scale insects display complete metamorphosis, whereas female development is paedomorphic (adults resemble nymphs). A number of taxa display remarkable diversity in their genetic systems (e.g., parthenogenesis, hermaphroditism, and paternal genome elimination) as well as in chromosome number, sperm structure, and types of endosymbioses.

FIGURE 12 Hermaphroditic adult of the cottony cushion scale, Icerya purchasi (Monophlebidae), on Grevillea juniperina, New South Wales, Australia; note the long, fluted ovisac emanating from the ventral abdomen of the insect.
The name “scale insects” derives both from the frequent presence of a protective covering or “scale” and from the appearance of many of the female insects themselves. Most species produce a waxy secretion that covers the body either as a structure detached from the body (a scale or test) or as a secretion that adheres to the body surface. Some coccoid species have been used as sources of candle wax, lacquers such as shellac, or dyes. Scale insects are more diverse in terms of major evolutionary lineages (families), species richness, and morphology than any of the other sternorrhynchan groups.
Evolution and Classification
Scale insects have been assigned variously to 20 or more families, but the family-level classification is controversial. Often the Coccoidea is divided into two major, informal groups, the archaeococcoids (or archaeococcids) and the neococcoids (or neococcids). The extant archaeococcoids comprise the Margarodidae sensu lato (with about 440 species and now treated as 11 families including Margarodidae sensu stricto, Matsucoccidae, Monophlebidae, and Xylococcidae), Ortheziidae (ensign scales; almost 200 species), Putoidae (about 60 species of Puto), Carayonemidae (4 species), and Phenacoleachiidae (2 species). Collectively, the above 15 archaeococcoid families only number approximately 100 genera and 700 species. Some of the morphological features that define the archaeococcoids occur more widely in the Hemiptera and monophyly of archaeococcoids is uncertain.
The neococcoids, which comprise all of the other extant families (usually 17 are recognized) and most of the species of scale insects (about 7000), are a monophyletic group characterized by derived features, including a chromosome system involving paternal genome elimination, and loss of abdominal spiracles. Among neococcoids, most families (except the Eriococcidae) are well characterized morphologically. In contrast, relationships among families are largely unknown or not supported well by available data. The three largest families of neo-coccoids, in order of size, are the Diaspididae (armored scales; about 2400 species; Fig. 13), Pseudococcidae (mealybugs; about 2200 species; Fig. 14), and Coccidae (soft scales; about 1150 species; Fig. 15). The other neococcoid families are the Eriococcidae (felt scales; ca. 560 species), the three pit scale families—Asterolecaniidae, Lecanodiaspididae, and Cerococcidae—totaling about 350 spp., Kerriidae (=Tachardiidae, lac insects; about 90 species), Kermesidae (gall-like scales; about 90 species), Aclerdidae (about 60 species), and 7 smaller families, in order of size—Conchaspididae, Halimococcidae, Stictococcidae,

FIGURE 13 Adult female armored scales of Diaspis manzanitae (Diaspididae) on leaf of Arctostaphylos species, California, USA.

FIGURE 14 Adult female mealybugs of Dysmicoccus neobrevipes (Pseudococcidae) on unidentified plant, Thailand.
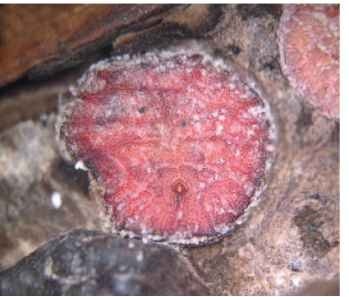
FIGURE 15 Adult female soft scale of Cryptostigma reticulolami-nae (Coccidae) from nest of Azteca ants in hollow stem of Cordia all-iodora in Mexico; the triangular anal plates are visible.
Beesoniidae, Dactylopiidae (cochineal insects), Micrococcidae, and Phoenicococcidae—totaling about 100 species.
The oldest described fossil scale insects are from the Lower Cretaceous but the group is at least of Triassic and probably of Permian age. Archaeococcoids comprised the earliest lineages with the neococ-coids apparently diversifying in conjunction with flowering plants.
Life History
The reproductive repertoire of scale insects includes hermaph-roditism, several kinds of parthenogenesis, and six major types of sexual chromosome systems. In the vast majority of coccoid species, the males are functionally (because of inactivation or elimination of paternal chromosomes), and sometimes actually, haploid.
Each individual female scale insect has four or five growth stages (Fig. 16) : the egg, two or three immature (nymphal) instars, and the adult (imaginal stage). The female either lays eggs (ovipary) in a cavity under her body or in a waxy covering (ovisac; Fig. 12) that may be attached to her body, or she retains the eggs in her reproductive tract until they are ready to hatch (ovovivipary). The mobile first-instar nymphs, called crawlers, are the main dispersal agents for Coccoidea; other immature instars generally are sessile. Adult females may live for an extended period (months to several years).
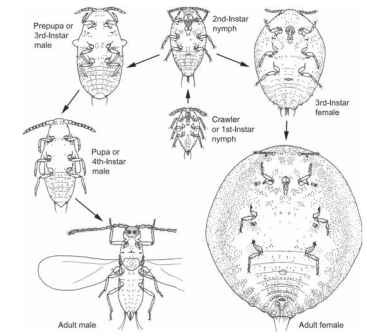
FIGURE 16 The life cycle of the Australian Acacia-feeding mealybug Melanococcus albizziae (Coccoidea: Pseudococcidae).
After hatching from the egg, male scale insects have a total of four immature or pre-imaginal instars including their own specially derived form of a complete metamorphosis (holometaboly) involving one or two pupa-like stages (Fig. 16). These are called the prepupa and pupa, and develop either under a scale cover or test or inside a waxy cocoon or test that is produced by the second-instar nymph. Neither the pupa-like instars nor the adult males feed; adult males are short-lived (at most a few days) and have limited time to seek out the sedentary females for mating.
Host-alternating life cycles, as seen in some aphids, are unknown. The number of annual generations varies among and often within species and ranges from fewer than one to up to seven or eight per year. Annual life cycles are common in cool-temperate regions but numerous generations per year, typical of many aphids, do not occur in scale insects.
Morphology
Morphologically, scale insects are among the most unusual of insects. There is a very marked dimorphism between the adult male and female and identification is based almost entirely on the long-lived adult female. Adult females (Figs. 12, 14-16) are saclike without a well-defined head, thorax, or abdomen, and they are paedomorphic, perhaps due to neoteny, that is, the adult resembles an immature individual. Adult females are wingless, may or may not have legs, but usually have well-developed mouthparts. The anus opens posteriorly and usually is surrounded by a chitinized ring and often anal plates (Fig. 15) or anal lobes flank the opening. Species that have a scale cover (e.g., armored scales; Fig. 13) usually incorporate in it the dorsal part of the cast-off cuticle from the previous growth stage. If the body wax is of the adhering type, then it may vary from a thin translucent sheet (many soft scales) to a thick, wet mass (e.g., wax scales), a cottony secretion (e.g., some monophlebids; Fig. 12 ) or a powdery, white dusting (many mealybugs; Fig. 14) . Species that produce waxy tests (e.g., felt scales) often also have a dusting of powdery body wax. Wax is produced by epidermal glands and is exuded from a great variety of cuticular pores and ducts, and sometimes also from glandular setae. Typically the body also is covered in setae that may be hair-like, spine-like, or other shapes, often with several types present on each species.
Adult males resemble small, delicate flies (Fig. 16) and have a distinct head, thorax, and abdomen. Most have a pair of membranous fore wings and a pair of vestigial hind wings (balancers or halteres), although adult males of some species are wingless. The mouthparts of the prepupa, pupa, and adult males are reduced or absent.
Crawlers (Fig. 16) are usually ovoid or elongate-ovoid and flattened dorsoventrally. Their antennae, legs, and mouthparts are well developed and various kinds of pores, ducts, and setae are present on the body and its appendages; often a fringe of setae surrounds the body margin.
Behavior and Ecology
The first-instar nymphs either seek suitable feeding sites on the natal host plant or disperse on the wind; some crawlers display behaviors that increase their chances of becoming airborne. Adult males probably locate their sessile conspecific females using sex pherom-ones but the presence of these chemicals has been demonstrated experimentally for very few species.
Scale insects primarily feed from either the phloem or parenchyma, and their host associations range from monophagous to polyphagous. Sap removal is the main cause of plant damage, but a few species (especially of mealybugs and armored scales) also transmit plant pathogens and/or toxins that may further reduce plant vigor and eventually kill the host. Furthermore, most scale insects (except armored scales and a few others) produce honeydew and are ant-attended; associations are usually facultative but a number of obligate ant-coccoid relationships have been described.
Curiously, cochineal scales (Dactylopius spp.) and certain mealybugs and lac insects have been used as biological control agents for particular noxious weeds; for example, cochineal insects can assist with the control of prickly pear cacti, Opuntia species.
Notable Pest Species and Their Control
Some scale insects are serious plant pests, especially of perennial agricultural plants. They can cause damage to nut and fruit trees, forest or plantation trees, glasshouse plants, woody ornamentals, house-plants, and sometimes to sugarcane and even grass in lawns. Pests are usually either polyphagous or oligophagous. The cryptic habits and small size of most scale insects means that they may not be detected until plant damage is substantial. Also, if populations on plants are low, they can be notoriously difficult to detect during quarantine inspections. Most pest scales belong to the Diaspididae, Coccidae, and Pseudococcidae, but a few significant pests belong to other families, such as the Monophlebidae (especially polyphagous Icerya species; Fig. 12), Eriococcidae, and Asterolecaniidae.
The most important predators of scale insects are ladybird beetles (Coccinellidae; especially species of Rodolia, Chilocorus, and Cryptolaemus). The main parasitoids of scale insects are chalcidoid wasps, especially species of Aphelinidae (e.g., species of Aphytis, Encarsia, and Coccophagus) and Encrytidae (e.g., species of Anagyrus, Leptomastix, and Metaphycus), although some scale insects are attacked by flies that may be either parasitic (e.g., Cryptochetidae) or egg predators (e.g., a few Cecidomyiidae).
