Sociality
Insect sociality refers to populations of insects that use some form of social behavior. Eusocial insects, for example, are true social species that possess the following three characteristics: cooperative brood care, overlap of two or more generations with offspring assisting with brood care, and reproductive division of labor. In a broad sense, the term “social” is often used for pre- and subsocial insects that have fewer than three of the characteristics of eusociality. In a strict sense, a social insect is usually understood to mean a representative of the ants, termites, vespoid wasps, and social bees, the true eusocial insects. Social insects are an important component of Earth’s biological diversity. E. O. Wilson has noted that eusocial insects (including termites, ants, bees, and wasps) make up a significant proportion (75%) of the world’s insect biomass. The evolutionary and ecological success of social insects is evident in both tropical and temperate ecosystems.
Sociality is a life history strategy that has evolved multiple times within and among diverse and distantly related insect taxa. Because of large population sizes, social insects collectively consume more energy and biomass than vertebrate animals. In tropical ecosystems, ants and termites play a major role in nutrient cycling and soil turnover. In 1990 B. Holldobler and Wilson noted that in tropical and temperate habitats, the amount of soil turnover attributable to ants and termites far outweighs the cycling done by earthworms. In the region of the Brazilian Amazon, ants release a significant quantity of atmospheric formic acid, which contributes to natural acidification of rainwater and weathering of limestone-based rock formations.
In eusocial insects, the ability to reproduce has been lost in worker and guard castes. Sterile workers care for the brood, provide nourishment, maintain environmental conditions (e.g., by thermoregula-tion) inside the nest, and remove waste. The female reproductives or gynes generally outlive several generations of the offspring and are protected and tended by workers in the confines of the nest. The presence of reproductive division of labor is vividly portrayed in social insects, with reproduction restricted to the queen, and nest or colony support being provided by the sterile worker and guard castes. With these three characteristics operating, an insect society is a highly organized, cooperative group of organisms that functions like a super-organism. Social and physiological homeostasis of the insect society is maintained through the integration of caste members through phe-romone, tactile, auditory, and sometimes visual communication.
The evolutionary patterns within the Hymenoptera are complex, and eusociality has evolved over eight times within the social bees. The Isoptera (termites) is the only insect order that is exclusively eusocial. Eusociality is rare but present in some aphids (Heteroptera: Cerataphidini) and thrips (Thysanoptera). In aculeate Hymenoptera, many examples of eusocial species are present and include all ants (Formicidae), one group of sphecoid wasps (Crabronini), vespoid wasps (Vespidae, Masaridae, and Eumenidae), and the Apoidea or social bees (Apidae: Bombinae, Apinae, Meliponinae, and Euglossinae).
DIVERSITY IN INSECT SOCIALITY
In 1923 W. M. Wheeler published one of the first contemporary treatments of insect sociality. In the 1960s and 1970s, usable classification schemes for insect sociality evolved from the works of C. D. Michener and E. O. Wilson. Wilson’s 1971 treatment of the social insects streamlined and established a standard vocabulary for describing the degrees of insect sociality, ranging from solitary to eusocial, with several different grades or intermediate forms (Table I ).
TABLE ISynoptic Overview of the Degrees and Characteristics of Sociality Derived from Wilson |
||
| Degree of sociality | Characteristicsa,b | |
| Overlapping generations |
Reproductive Cooperative castes brood care | |
| Quasi-social Semisocial Eusocial |
+ | + + + + + |
aPlus sign ( + ) denotes presence and minus sign ( —) indicates an absence of a characteristic.
bSolitary, subsocial, and communal insects do not have overlapping generations, reproductive castes, or cooperative brood care.
Presocial Insects: Subsociality
Most insects lead solitary lives, with many species forming simple aggregations during mating or at commonly used food sources. Sociality appears in insects as a departure from a solitary life history strategy. Presocial insects have one to two characteristics of sociality. One subgroup of presocial insects includes insects that provide limited parental care to their offspring. Labeled as subsocial, these species protect or shelter their eggs, nymphs, or larvae; eggs and larvae may be given parental care in an enclosed nest.
Most subsocial insects provide care of offspring in the absence of a nest or shelter. Many examples of subsociality are known from the true bugs (Heteroptera). For example, in some belostomatid genera (Belostoma, Abedus) the female lays her eggs on the male’s back, and the male carries the eggs until nymphs hatch. Males of the belostomatid Lethocerus americanus assist the female with active guarding of the eggs that are laid on aquatic vegetation. Parental care of eggs is also known in water striders (Gerridae) and leaf-footed bugs (Coreidae). Active guarding of eggs and newly hatched nymphs is known in the Acanthosomatidae, Pentatomidae, Tingidae, Reduviidae, and Scutellaridae. Usually, the female guards the clutch and covers the first instars when a predator approaches. The nymphs often orient themselves toward the parent to facilitate concealment.
More examples of behaviorally advanced stages of offspring care can be found in the orders Heteroptera and Coleoptera. Treehoppers (Heteroptera, suborder Auchenorryncha; Membracidae) also exhibit parental behaviors toward early instars. R. Cocroft, for example, observed female thornbugs (Umbonia crassicornis) straddling egg clutches to prevent parasitism and creating feeding slits on plant stems for hatchlings. Additionally, the mother actively herds wandering nymphs to keep them near or around the feeding slits and emits alarm calls to alert offspring to a predator’s presence.
Within the Coleoptera, highly developed forms of parental behavior and nest making have been discovered in the Silphidae and in the dung-feeding Scarabaeidae. Known as burying beetles, species of Nicrophorus (Coleoptera: Silphidae) provide provisions and actively feed newly hatched offspring through regurgitation. After discovering a small animal carcass, Nicrophorus parents process the carcass into a congealed ball of putrefying flesh. After burying the provision, the female chews a shallow depression into the mass, where she deposits her eggs. To solicit parental feeding, the larvae raise the anterior portions of their bodies and wave their thoracic legs. The female parent then regurgitates a small droplet of food material, which is devoured by a larva. In some Nicrophorus species, males also participate in larval feeding but less frequently than the female. Parental feeding in Nicrophorus is essential for development of the young: removal of the female parent inhibits metamorphosis to the adult.
G. Eickwort and Wilson categorize most bark and ambrosia beetles as subsocial or colonial insects. Members of the Scolytidae and Curculionidae (Platypodinae) have elaborate life history strategies that enable them to evade a host tree’s defense mechanism. The scolytid beetle Xyleborus produces communal brood chambers where all life stages reside. In similar manner to Hymenoptera, these xyleborines also have haplodiploid sex determination. Another similarity between xyle-borines and eusocial Hymenoptera is the presence of a biased sex ratio in favor of females. Female offspring assist in cultivating ambrosia fungi, which provide the cellulase necessary for digestion of wood fibers.
Parasocial Insects: Communal Societies
“Parasocial” refers to species that exhibit communal, quasi-social, and semisocial behavioral patterns with some form of generation interaction between generations. Communal species include insects of the same generation that share a nest site without any form of collective offspring care. A communal life history strategy is not just an aggregation: it may involve complex forms of communication and offer some distinct advantages over a solitary life history strategy. Communal aggregations enhance accessibility to food materials that are difficult to process, facilitate thermoregulation, and offer protection from predation. Examples of communal social behavior in bees can be found in the Andrenidae, Megachilidae, and several subfamilies of the Halictidae. Multiple females construct a large, composite nest, but each female tends only to her own nest cells. This produces a nest having a single nest opening that is more easily defended by multiple female bees. Many different species of beetles have communal life history strategies. Some species of scolytid bark beetles are exemplary communal insects.
Communal behaviors in larval insects can be found in tent-making caterpillars (Lepidoptera: Lasiocampidae) and some of the sulfur butterflies (Lepidoptera: Pieridae), silk moths (Lepidoptera: Saturniidae), nymphalid butterflies (Lepidoptera: Nymphalidae), and tussock moths (Lepidoptera: Lymantriidae). Batch laying of eggs predisposes some larval insects for communal life history strategies, but other species disperse from a common oviposition site to lead solitary lives. Although complex social interactions between group members are limited in many species of caterpillars, some species utilize central-place foraging that requires an extensive communication network within the caterpillar community. Central-place foraging is not tied universally to web making, but instead is scattered throughout the lineages of Lepidoptera. In 1925 F. Balfour-Brown described a model for the evolution of elaborate caterpillar web dwellings from a mat of silk threads or carpet web. This model suggests that carpet webs, the ancestral condition, further evolved with caterpillars that used feeding webs and later to those that used domicile or home webs.
In the jack pine sawfly, Neodiprion prattti banksinae (Hymenoptera: Neodiprionidae), communal feeding facilitates the procurement of nutrients from tough waxy pine needles. The collective efforts of many larvae chewing over the same area on a pine needle reduces the amount of time needed to break through the cuticle. Other species of larval sawflies and Lepidoptera use group defense postures in which the aggregation moves in a synchronized manner to thwart a predator. The sawflies Neodiprion sertifer and Diprion pini use synchronized head jerking and twitching to repel both egg-laying ichneumonid wasps and birds. To be effective, these protective behaviors require a coordinated group response. Similar defense strategies are also known in the woolly or eriosomatid aphids in which a coordinated waving of waxy filaments may intimidate predators.
Parasocial Insects: Quasi-Social Societies
Unlike communal insects, quasi-social insects have some cooperative brood care while sharing a common nest site. Female quasi-social bees participate in nest guarding and assist with building and stocking nest cells with provisions. In 1967, R. B. Roberts and C. H. Dodson discovered that multiple female orchid bees (Apidae: Euglossinae) often occupy a shared nest site, with the number of females present usually exceeding the cells available for egg laying. This finding suggests that euglossine bee societies have a form of cooperative brood care. Temporary quasi-sociality is also known in a few species of polis-tine wasps with age polyethism. Young females begin their adult lives as foragers and assist with nest provisioning. Later on, these individuals become egg layers in the common nest site.
Parasocial Insects: Semisocial Societies
C. D. Michener has stated that semisocial insects have an additional characteristic of sociality over the quasi-social species in that the former also have some form of reproductive caste that is cared for by a worker caste. Variation in ovary maturation in spring halic-tine bee populations suggests that the semisocial condition can arise spontaneously within a mixed assemblage of reproductives of different stages of maturity. Similar patterns of seasonal semisocial behavior occur in some Polistes wasps.
Eusocial Insects
The fundamental difference between semisocial and eusocial insects is that eusocial insects have overlapping generations that feature cooperative brood care by the offspring. Eusociality has evolved multiple times within insects and first appeared in the termites. Eusociality in lower termites (Hodotermitidae, Termopsidae, Kalotermitidae, Mastotermitidae, Rhinotermitidae) is associated with trophallaxis and enables all individuals of the nest to be inoculated with gut symbionts necessary for the digestion of cellulose. Trophallaxis also serves to transfer chemical communication in lower and higher termites. Higher termites such as the Termitidae consume sources of cellulose other than wood, lack flagellate endosymbionts, and use anal trophallaxis for transferring chemical cues.
N. Lin and C. D. Michener proposed two distinct pathways of character evolution in social insects. Most bees are believed to have evolved eusociality through a parasocial sequence. Ancestral social bees used a communal social strategy with cooperative nest construction. Later generations switched from a communal to a quasi-social strategy incorporating cooperative brood care within an annual life cycle. The semi-social stage followed the quasi-social stage, with a well-defined worker caste providing cooperative brood care. Fully evolved eusocial species then acquired the characteristic of overlapping generations.
Ants, termites, vespid wasps, and some social bees are believed to have used a subsocial sequence for the evolution of eusociality. The subsocial sequence for the evolution of eusociality is based primarily on the degree of brood care by the female with respect to the age of the offspring. In basal or ancestral taxa, brood care was given only to early instar offspring, with the mother abandoning the young well before maturity. In stage two, brood care is extended to the time during which the young reach maturity. In the final sequence of events, some of the brood become permanent, sterile workers and provide cooperative brood care to the female parent.
Eusociality has also evolved in some gall-forming thrips and aphids. These insects have recently been classified as eusocial because of the presence of a sterile guard caste. However, this form of restricted eusociality differs in important ways from the eusocial condition of Hymenoptera. The guard caste is the only nonreproductive caste in aphid and thrips societies, and the guards do not care for the young. In aphids, this is partly because aphid offspring are precocious like small versions of the adult, they are motile and able to feed themselves.
B. Crespi discovered eusociality in several species of phlaeothripine gall-forming thrips. In these eusocial thrips, the first generations of adults develop into sterile soldiers and are used for defending the gall against invasive species such as ants. These Australian thrips reside in Acacia leaf galls or leaf mines and utilize social behaviors for defense of the domicile. The guards also assist with domicile construction. Morphological specialization associated with the guard caste in euso-cial thrips includes enlarged prothoracic legs that are used to attack invaders. The prothoracic femora may be expanded and adorned with robust spines. Mortality in the soldier caste is high.
S. Aoki along with Y. Ito have shown that soldier castes are also present in the Cerataphidini or bamboo aphids (Hormaphididae) and in the gall-forming pemphigid aphids. Ito found that some of the Cerataphidini produce a sterile guard caste that defends the offspring. In Ceratovacuna and Pseudoregma, members of the soldier caste are adorned with sharply pointed spines on the head and are used for colony defense on secondary hosts. In Pemphigus obesin-ymphae, sterile guards are also used for protecting the domicile. The guards are larger than the nymphs and kick at invading predators with their long legs. In social aphids, the guard caste never assists with brood care but serves only to protect the colony from preda-tion. Parthenogenesis is a reproductive mode employed by aphids, but how the soldier caste is determined is unknown.
In 1992 D. S. Kent and J. A. Simpson discovered a eusocial ambrosia beetle (Coleoptera: Curculionidae). A member of the Platypodinae, Austroplatypus incompertus lives within heartwood galleries of Eucalyptus. Platypodine beetles like A. incomperus feed on ambrosia fungi, and nonreproductive females tend the galleries and process fungal mycelia. Kent and Simpson found that established colonies older than 2 years were composed of a single fertilized female, and up to five unfertilized females, which helped maintain the gallery structure and provide protection from predators. The presence of overlapping generations, reproductive/nonreproductive castes, and cooperative brood care within a shared domicile provide evidence for eusociality in these ambrosia beetles.
Within the Hymenoptera, eusociality is found in all ants (Formicidae), vespid wasps (Vespidae), and some bees (Apidae). Members of the subfamilies Bombinae, Meliponinae, and Apinae are eusocial. Sex determination in Hymenoptera follows Dzierzon’s rule, first shown by J. Dzierzon in 1845 with honey bees and now termed haplodiploidy. Male progeny are produced from unfertilized eggs through a process called arrhenotoky. In many species of social Hymenoptera, males can be produced at the appropriate time from unmated females within the colony. Workers will sometimes undergo facultative thelytoky to produce diploid eggs. Automictic parthenogenesis produces the diploid eggs in queenless hives of A. mellifera capensis. This form of thelytoky is uncommon and is restricted to certain subspecies of the honey bee.
COLONY SIZE AND LONGEVITY IN EUSOCIAL INSECTS
Insect societies may range in size from fewer than 10 to over 3 million adults. Some species of termites (families Termitidae, Mastotermitidae, and Rhinotermitidae) have colonies with millions of members. Colonies of vespid wasps can have 100 to over 5000 members; colonies of the Allegheny mound ant, Formica exsectoides, can exceed 40,000; and nests of A. mellifera usually contain between 20,000 and 80,000 individuals.
Species that build nests located on or in the ground tend to have more colony members than species that use more restrictive nest sites. Ground-nesting vespid wasps, for example, usually have larger colony sizes than wasps that build arboreal carton nests. Species that have a large colony size generally tend to have more complex social behaviors than species with small colonies. The correlation of increased colony size with highly evolved social behavior suggests that complex behavioral integration is necessary for colony survival.
Although most data gathered on longevity have been derived from laboratory populations, the female reproductive or queen of some eusocial insects can live for more than 10 years. Well-protected inside the nest, most queens are tended by a court of workers and insulated from the hazards of everyday life. Workers have significantly shorter life spans and are quickly replaced with newer offspring. Attrition is high among workers, and approximately 1% of the worker caste of the army ant Eciton hamatum is lost daily. Unlike the queen, workers are exposed to many hazards, and activity outside the nest contributes greatly to worker mortality.
ROLE OF ALTRUISM IN INSECT SOCIALITY
Kin selection is the form of natural selection essential for explaining how the characteristics of eusociality evolved. In 1964 W. D. Hamilton published a mathematical model explaining the economic basis for self-sacrifice as a result of altruism. Hamilton’s rule, C/B < r, suggests that the ratio of the cost C of sacrifice between two closely related individuals to the benefit B of the receiver r must be smaller than the degree of relatedness between the two individuals. Thus, the inclusive fitness of a group of related individuals is the amount of individual and kin selection. The application of Hamilton’s rule to social Hymenoptera, which follow Dzierzon’s rule with haploid males and diploid females, is illustrated by the following example. A female offspring inherits 50% of its genome from its father and 25% of its genome from its mother. Hamilton’s rule predicts that sisters produced from the same mating should tend to sacrifice themselves for each other because they share 75% of their genetic material with each other and 50% of their genetic material with their mother. Thus, the benefit for an altruistic behavior does not have to be high to spread throughout a population.
In 1977 H. E. Evans proposed that selection factors other than kin selection could explain the evolution of altruism and social behaviors in Hymenoptera. Extrinsic factors such as nest parasitism are just as plausible as kin selection for the evolution of altruism. Evans noted that in general, polygynous (multiple queens per nest) wasp species are more common than monogynous (single egg-laying queen per nest) species. Evans hypothesizes that polygynous nesting strategies are ubiquitous because they are necessary for protecting the progeny and provisions of the extended family group from nest parasitism. Likewise, polygynous nests can provide many foragers for provisioning the nest as well.
Although altruism can be detrimental to the individual, advantages to the group or colony are many. Most social insects utilize group defensive strategies when the nest or colony is under attack. Aggressive defense strategies observed in social Hymenoptera, social aphids, and termites demonstrate that defensive behaviors are usually detrimental to the defenders but facilitate survival of the reproductives and brood within the nest. Self-sacrifice occurs frequently in social insects. In honey bees, for example, the barbed sting of the worker bee eviscerates and kills the worker after stinging, suggesting that the cost of altruism to the individual is high but trivial to entire colony. In termites, guards will position themselves closest to the source of danger when provoked, whereas other colony stimuli are generally ignored.
Although queen adoption is most often associated with colony multiplication, Wilson believes that the genetic advantages for this strategy are clear. Increasing the number of queens in an isolated colony serves to increase the effective population size while incorporating more altruistic traits into the colony. However, polygyny in ants is not restricted to rare, isolated populations, but can also be found among ubiquitous ant populations as well.
CASTES IN SOCIAL INSECTS
The caste system and polyethism determine the division of labor in insect societies. Castes divide colony members into distinct functional roles within the insect society. Specialized caste members conduct such tasks as defense, brood care, nest construction, thermoregula-tion, provisioning, and egg laying. Morphological specialization or caste polyethism is a key feature of caste structure. The role of a caste member may also change with age (age polyethism).
Morphological specialization associated with caste polyethism is often the result of allometry and usually is tightly coupled with behavioral changes. Allometry or differential growth rates can provide dramatic changes in morphology with simple change in relative growth rates (Fig. 1). Allometric growth provides termite or ant guard castes with hypertrophied mandibles that are used for defense. The head capsule, which may also be enlarged relative to other body parts, accommodates the mandibular muscles used for biting and dismembering enemies.
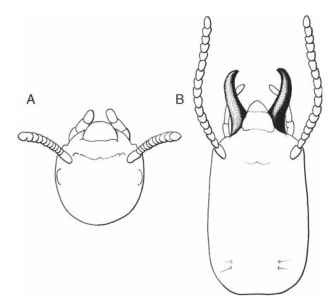
FIGURE 1 Cranial morphological differences in worker and soldier or guard termite castes. In Reticulotermes flavipes, workers (A) have short chewing mandibles concealed beneath the clypeus and soldiers (B) have an elongated, enlarged head with prominent mandibles.
Overall change in body size also can be important in defining the queen caste. In most eusocial insects, the queen is larger than nonreproductives. The abdomen, which houses the ovaries, may be grotesquely enlarged in mated termite queens and some ant queens. Such enlargement (physogastry) also is known in replete ants, which gather and store honeydew in the crop.
The castes of typical social insects can be categorized according to the following classification scheme. Within social Hymenoptera, guards serve as nest defenders and are equipped with well-developed mandibles and potent stings. Workers, the most ubiquitous caste, help with nest construction and repair, thermoregulation, provisioning, and brood and queen care. The queen makes up the reproductive caste, and her egg-laying activity provides the fecundity for maintaining the colony. Most of the members of an insect society are workers, and high numbers of this caste are necessary for maintaining the physiological homeostasis of the domicile.
Many differences are evident between the caste system of termites and social Hymenoptera. In lower termites, the second instars function like workers and are actively involved in nest maintenance. Because, at least in part, of their hemimetabolous development, larval termites are active and also take care of themselves as well. Unless destined to become guards, most apterous termite larvae remain as workers throughout their lives. Termite castes differ from social Hymenoptera castes in that they contain both sexes, with reproduction restricted to the fertilized queen (Table II). Once established, termite colonies can replenish reproductives from several distinct supplementary reproductive castes. Within the Termitidae, adultoid reproductives can become functional reproductives and replace lost reproductives. Nymphoid reproductives (larvae that possess short wing pads) can become fully functional supplementary reproductives. Ergatoid reproductives are neotenic, apterous individuals with pigmented exoskeletons. Inhibitory pheromones released by functional reproductives prevent the supplementary reproductives from attaining functionality.
In honey bees, morphological and behavioral differences in castes are regulated by endocrine control of development, dietary differences of larval workers and queens, and queen pheromonal control. Hormonal developmental control is associated with the activity of juvenile hormone (JH) on target tissue sensitivity to ecdysteroids. This modulates or controls reproductive tissue differentiation in developing larvae. In addition, JH may inhibit the release of ecdysteroid and determine the duration of the last instar feeding stage. In developing queens, JH may inhibit ovariole cell death, a feature common in developing workers. Other hormones also influence ovariole development in larval queens. Makisterone A, an ecdysteroid secreted by the prothoracic glands, promotes ovariole differentiation.
Dietary differences are correlated with reproductive caste development in honey bees. Honey bee worker larvae are fed pollen and honey, whereas queen larvae are fed royal jelly. The hypopharyngeal and mandibular glands of workers produce royal jelly. The production and feeding of royal jelly to select larvae is associated with lowered amounts of queen substance, reduced queen vitality, or queen death; the construction of queen cells is also debilitating to the queen. Different proportions of proteins, lipids, carbohydrates, and trace compounds are apparent in honey and royal jelly, but the active ingredient in royal jelly responsible for queen development is still unknown.
Pheromonal control is also evident in caste formation in honey bees. Queen mandibular gland secretion reduces the amount of JH in developing worker larvae, stimulates worker foraging, promotes queen tending, and inhibits swarming behavior. Through modulation of JH titers in developing workers, queen substance inhibits ovariole development.
Examples of age polyethism are frequent in social Hymenoptera. In worker honey bees and ants, temporal changes in maintenance behaviors are associated with age. Young workers take care of the queen and feed the larvae, whereas older workers conduct most of their activities outside the nest. Behavioral changes in honey bees also correlate with the thickness of the mandibular, hypopharyngeal, postcerebral, wax, and thoracic glands. The nursing period of worker bees coincides with the maximum development of mandibular and hypopharyngeal glands at between 5 and 10 days of age.
COMMUNICATION IN EUSOCIAL INSECTS
The functional integrity of an insect society is established through complex forms of communication. Communication pathways, either chemical, visual, or acoustic, can be used to convey alarm, simple and multiple attraction for assembly, recruitment to a new domicile or food source, grooming, trophallaxis and exchange of food material, facilitation or inhibition of group or colony effects, nestmate recognition, and caste determination. Trophallaxis and exchange of food material also function as mechanisms for the transfer of chemical information between members of a eusocial insect colony. Sensory cues of many different types are used for communication in social insects, and chemical and acoustic cues have been discovered in termites and social Hymenoptera. Visual communication is limited but, according to Karl von Frisch, plays an important role in the waggle dance of honey bees, in which the bees indicate the location of a food resource through a series of stereotypical movements. Chemical communication is believed to be important for maintaining caste structure, nestmate recognition, and establishment of the queen in an insect society. Chemical signals have an immediate effect that typically ends immediately after the chemical has diffused away from the sensory receptors. Some chemical and acoustic cues have also been implicated for initiating defensive and flight behaviors in social insects.
Queen Dominance and Pheromones
Queen dominance in higher social insects is essential for controlling nestmates and is maintained with a variety of chemical cues in social Hymenoptera. In honey bees such as A. mellifera, the mandibu-lar glands and Koschevnikov’s gland produce the components of queen substance. In A. mellifera, mandibular gland secretion is a pheromone blend composed mostly of 8-hydroxyoctanoic, (E)-9-oxo-2-decanoic
TABLE IIGeneralized Classification Scheme of Caste Structure in Termites Derived from Wilson |
||
| Caste | Morphological features | Roles |
| Larvaea Worker Pseudergate Soldier/guard Primary reproductivesb |
Usually apterous (wingless) and lack reproductive and guard caste structures Brachypterous larvae (wing pads present) are known as nymphs Absence of compound eyes and ocelli; apterous, with reduced pterothorax, well-developed jaws Regressed nymph, found only in lower termites Enlarged mandibles, hypertrophied head glands, head Queen or male derived from alate adults |
May develop into workers or guards May develop into functional reproductives Nest construction, sanitation, provisioning, brood care May develop into secondary reproductives, soldiers Defense Colony founders |
aImmature termites known as larvae are further grouped by the presence or absence of distinct wing pads. bPrimary reproductives become dealate reproductives after shedding their wings.
(9-ODA), (E)-9-hydroxy-2-decanoic, 10-hydroxydecanoic, and (E)-10-hydroxy-2-decanoic acids. As a newly emerged queen ages and her attractiveness to workers increases, the relative concentrations of these organic acids change. Worker attractiveness is maximized after the queen becomes mated. Although 9-ODA alone does not cause worker court formation, when 9-ODA is supplied with mandibular gland secretion, court formation occurs. The pheromone 9-ODA is also associated with the inhibition of queen-rearing behaviors and gonad development in worker bees. A possible mode of action of 9-ODA is to reduce the quantity of gonadotrophic hormone so that ovary development is inhibited in worker bees.
Court attraction, in which is a group of workers gather around and tend the queen, is controlled via glandular secretion. Koschevnikov’s gland secretions from mated queens are correlated with court attraction. This gland plus mandibular gland secretions may serve to provide an olfactory stimulus for court formation from workers at short distances from the queen. Virgin queens do not form worker court aggregations as readily as do mated queens, which suggests that Koschevnikov gland secretions alone may not be responsible for court formation. Abdominal tergite gland secretions also work as a contact pheromone and facilitate court formation after workers touch the queen’s abdominal tergites with their antennae.
Colony Odor
Nest odor is believed to be important for nestmate recognition in social insects. Sources of nest odor are not clearly defined, but a combination of genetic and environmental factors may be important sources of colony odor. Environmental odors from shared foods like nectar or pollen may contribute to the uniqueness of an individual colony’s odor. Likewise, food transfer in honey bees may facilitate the spread of environmental odors to all members of the hive or nest. The waxy cuticle of honey bees may also trap environmental odors from the atmosphere of the nest. Cuticular hydrocarbons have been implicated as a means for nestmate recognition in Polistes wasps (Hymenoptera: Vespidae).
Queen odors are genetic factors that are believed to be a major component of colony odor. M. D. Breed provided some evidence to support this hypothesis. In 1981 Breed published some research findings suggesting that small groups of worker honey bees are more likely to accept a sister queen over an unrelated queen. Further research has demonstrated that resident bees are able to detect and recognize foreign queens raised under the same environmental conditions. This provides additional support for the presence of queen odor as a component of colony odor.
Trail Pheromones
Trail pheromones are used for recruitment, for marking pathways to resources, and for indicating resource richness. Often released with alarm pheromones, trail pheromones enable guards to aggregate around a nest invader. Furthermore, short-lived and persistent trail pherom-ones are known. In ants, trail pheromones are produced in the hindgut, Dufour’s gland, the venom gland, and the tibial, tarsal, and abdominal glands. In some myrmecine and ponerine ants, the venom gland is the source of trail odors. Odor trails produced by termites originate from the abdominal sternal glands. In Zootermopsis nevadensis, sternal gland secretions deposited on a trail from a breached or damaged nest wall to the interior of the nest draws workers toward the area for nest repair.
Odor trails emitted by forest-inhabiting bees provide three-dimensional information on resource location. Some large sting-less bees of the genus Trigona produce strong odor chemicals from enlarged mandibular glands. Trigona forage in tropical forests and utilize mandibular gland secretions to cue in other foragers to nectar sources. Foraging Trigona will release more trail pheromone near a food source, thus indicating proximity to the food source for other foragers. The trail pheromone components of T. subterranea include both E- and Z-citrals. Differences in pheromone composition between species may reduce interspecific trail following in stingless bees.
Alarm Pheromones
Alarm pheromones are volatile organic molecules of low molecular weight that diffuse rapidly, forming a concentration gradient away from the signal source. Alarm pheromones initiate arousal, defensive, and assembly behaviors. Alarm pheromones are often not species specific in social Hymenoptera and termites, but the same pheromone often initiates different behaviors in different species. For example, the mandibular gland secretion 4-methyl-3-heptanone is associated with digging behavior in Pogonomyrmex species, but in high concentration causes repelling behaviors in the Texas leafcutting ant Atta texana.
Contextual responses to alarm pheromones are correlated with the proximity of the nest to the signal’s source. Flight occurs when alarm pheromones are perceived far from the nearest nest opening. When these substances are released near the nest, colony members become defensive. Aggregation, defensive postures, and frenzied excitement are often observed in agitated social insects.
Alarm pheromones are often coupled with other glandular secretions such as trail pheromones. Most alarm pheromones originate from the exocrine mandibular gland. Mandibular gland secretions produce a variety of responses that include excitement, attraction, and threat posturing in different species of ants. Other glands associated with alarm signals in ants include Dufour’s gland and the anal gland. In honey bees, isoamyl acetate is secreted by the lining of the sting pouch and generates attraction and investigation behaviors. After stinging, honey bees leave the sting and associated glandular components in the adversary’s skin, which releases more volatile alarm pheromones for attracting more defenders to the vicinity. The mandibular gland in honey bees secretes 2-heptanone, which initiates alarm behaviors.
The cephalic gland secretions found in termite soldiers are believed to initiate aggressive excitement behaviors in other soldiers and alarm in nonsoldier nest members. Known organic compounds produced by termite cephalic glands include limonene, terpinolene, and a- and 3-pinenes.
Acoustic Communication
Termites and ants also use some acoustic alarm signals, and some vespid wasps employ sound to alert offspring to the presence of provisions. Acoustic communication in social insects is conveyed through the substrate, not through the air. Acousticosensory organs present on the legs of social insects detect vibrations transmitted through the substrate.
Ants use subgenual organs and campaniform sensilla located on the legs to detect acoustic signals. Among carpenter ants, the subgen-ual organ is sensitive to frequencies ranging from 1.5 to 3 kHz. In the leafcutting ant, Atta cephalotes, campaniform sensilla function as a sound-detecting organ. Sensilla campaniformae are located near the distal end of the trochanter in A. cephalotes. The sensitivity of cam-paniform sensilla to sound waves varies from the anterior to posterior legs, with the anterior legs being the most sensitive to vibration.
Acoustic signals in ants are generated by stridulation or by tapping the gastral segments on the substrate. Stridulatory files are found universally in pseudomyrmecines, in over 80% of all studied myrmecines, and in nearly half of all studied ponerine ants. Stridulatory files when present are located at the junction of the abdomen (fourth abdominal tergite) with the postpetiolus. Buried or confined ants use stridulatory files to generate acoustic signals through the substrate for initiating digging behaviors in other workers.
Formicine ants are believed to have secondarily lost their stridula-tory files and none use different forms of auditory communication. Camponotus (carpenter ants) use a combination of mandibular and gas-tral tapping when disturbed. Response to these alarm cues depends on the state of arousal in the signal receiver. In some cases, the tapping behavior causes some ants to lie motionless, presumably to make them less visible to predators, while more agitated ants orient toward the source of the signal and approach it. Outside the nest, tapping enhances the stimuli associated with disturbance and functions as a danger alarm.
Similar tapping behaviors have also evolved in some vespid wasps but not as an alarm cue. Gastral vibration is believed to signal food or provisioning to larvae. The abdomen or gaster is rapidly tapped on the surface of the comb when provisions are brought into the nest. In Vespa tropica, foundresses tap their legs on the nest comb to signal the presence of provisions to hungry larvae. In this species, the sounds produced by leg tapping are loud and audible to humans standing at least 1 m from the nest.
In termites, sounds are generated by rapidly tapping the head or abdomen against the substrate. Head banging in Zootermopsis is audible as a rustling sound and is believed to function either as a substrate-transmitted alarm call or as a defensive behavior meant to scare intruders away from the nest. Research on the response of termite subgenual organs to the vibrations generated by head tapping behavior (1 kHz) suggests that these acousticosensory organs are highly sensitive to that frequency.
INQUILINES AND NEST PARASITES OF SOCIAL INSECTS
Eusocial insect nests often harbor guests that take advantage of the food resources and microclimates inside the nest chambers. Arthropod ectosymbionts of social insects are numerous and include some species of mites, spiders, millipedes, isopods, and insects. Most orders of insects contain some ectosymbiotic species. Some nest invaders, such as wax moths (Lepidoptera: Pyralidae), feed on the nest structure of honey bees, whereas others may feed on the young, stored provisions, or scavenge from the debris.
Some nest owners receive a benefit (mutualism) for harboring another species. Trophobionts like aphids (Heteroptera: Aphididae), scale insects (Heteroptera: Coccidae), and mealybugs (Heteroptera: Pseudococcidae) are often protected and sheltered by ants. In return, ants receive from these insects honeydew, a sugary fluid, while providing protection and shelter to these guests. Some have suggested that the posterior morphology of aphids mimics the head region of an ant, with honeydew secretion simulating the transfer of food between ant workers.
A diverse group of guests is usually encountered with army ant colonies. R. D. Akre and C. W. Rettenmeyer studied the association between ectosymbiotic staphylinid beetles and army ants. These beetles have evolved two distinct behavioral strategies to gain access to the resources provided by army ant colonies. Specialized species such as Ecitomorpha and Ecitiosus are mimetic forms that live within the bivouacs of army ants. They are highly integrated into army ant societies and die if removed from the confines of the colony. Often associated with the larvae or booty, these staphylinid beetles tend to move together within an emigrating column of ants. Phoresy is common, with army ant workers transporting and moving these staphylinids to new bivouacs. Microdonia uses a generalized strategy for exploiting army ants. The association between generalists and their host ants is less integrated than specialized species. They usually frequent the periphery of an army ant colony and will often attack wounded or dying ants. The ants will sometimes attack and attempt to drive these insects away from the nest area.
Evolved mechanisms that enable symbiotic associations between symphiles and their hosts may include behavioral and chemical mimicry. Many symphiles can attract hosts and through appeasement gain access to the nest. B. Holldobler was one of the first researchers to discover that scents are important for attraction of worker ants to larval Staphylinidae. Some researchers believe that trichome secretions have an intoxicating effect and cause disorientation in host species. The reduviid bug Ptilocerus ochraceus, an ant predator, uses a similarly acting substance to attract and paralyze Dolichoderus ants. This bug presents its abdominal trichomes to an ant, which begins to lick the trichome hairs leading to intoxication.
The variety of interactions between different species of ants is extensive. Interactions range from coexistence to true parasitic relationships. In plesiobiosis, distantly related ant species can coexist with minimal interactions between closely neighboring nests. Some ant species rely on cleptobiosis or thievery to acquire food or refuse from nests of ants of other species. Cleptobiotic species reside in separate domiciles from their hosts. Lestobiosis differs from cleptobiosis in that one species will actually invade the host’s nest to prey on the young or food cache. For example, ants of the genus Carebara nest within the walls of termite nests and are believed to prey on termites.
Mixed species colonies usually involve some form of social parasitism. Temporary social parasites were first recognized by Wheeler in 1904. Ants in the Formica microgyna group utilize this type of life history strategy. A newly fertilized queen enters a host colony and coerces the host workers to take over the nest through assassination of the host queen. Gradually, the parasitic queen’s offspring replace the host workers through attrition.
Slave-making ants invade a host nest and steal pupae for incorporation in their own nests. After eclosion, the slaves work as foragers and as nest constructors, and conduct brood care for the slave makers. Slave makers parasitize other ant species that are close relatives. Some species of Polyergus use Formica species for slaves, Formica species of the sanguinea group typically use other Formica species as slaves.
Permanent parasitism or inquilinism occurs entirely inside the host’s nest. In some species, workers are present but have limited behavioral roles within the host’s nest. For example, Teleutomyrmex schneideri no longer has a worker caste and represents a highly refined form of inquilinism. T. schneideri lives its entire life within the nest of Tetramorium caespitum, a close relative of T. schneideri. The queens of T. schneideri are smaller than the queens of T. caespitum and are morphologically patterned to ride on the backs of the host queen. Teleutomyrmex queens release attractants, from cuticular glands of the thorax and petiole and are eagerly tended by the host’s workers.
