Siphonaptera
(Fleas)
The Siphonaptera are laterally compressed, wingless, holom-etabolous insects. The order contains approximately 2575 species. All species are parasitic in the adult stage and possess mouthparts modified for piercing and sucking, highly modified combs and setae on their body and legs, and legs that are modified for jumping. Some species are vectors of disease, and current research is providing important insights into flea phylogeny and evolution. Krasnov (2008) has compiled a discourse on the functional ecology of fleas, and the authors would suggest that this reference is the most exhaustive ecological treatment of Siphonaptera to date.
EARLY RESEARCH ON FLEAS
Fewer than 100 species of fleas had been described prior to the end of the 19th century. In 1898, it was demonstrated that fleas were capable of transmitting plague organisms and, later, murine typhus, which stimulated a frenzy of flea studies. In the early 20th century,
A. C. Oudemans, Julius Wagner, Nathan C. Rothschild, and Karl Jordan made major contributions. Karl Jordan is recognized as “the father of flea systematics.” From 1953 to 1971, G. H. E. Hopkins and M. Rothschild published a comprehensive five-volume series on flea systematics, and three additional companion volumes were published for the remaining families by Mardon in 1981; Traub, Rothschild, and Haddow in 1983; and Smit in 1987. Robert Traub described 153 flea taxa and provided critical insights into flea phylog-eny, convergent evolution, and zoogeography. Today, there are only a few flea specialists with knowledge of the global flea fauna.
FOSSIL RECORD
Fossils of fleas are extremely rare. Three extinct species (Palaeopsylla baltica, Palaeopsylla dissimilis, Palaeopsylla klebsiana) have been reported from Baltic amber, and a single specimen (Pulex larimerius) has been reported from Dominican amber. The former three species belong to an extant Palaearctic genus, whereas the latter belongs to an extant genus in which five species are confined to the Nearctic and Neotropical regions, and a sixth is cosmopolitan (Pulex irritans). Several other extinct fossil specimens representing the South American family Rhopalopsyllidae exist, but have never been adequately studied and published.
ZOOGEOGRAPHY
Current distribution of flea families suggests an ancient origin, possibly in the Jurassic period (> 140 mya). In general, families endemic to the boreal continents (North America, Europe, Asia) are predominantly CeratophyUidae, Hystrichopsyllidae, Leptopsyllidae, VemipsyUidae (Holoarctic), Coptopsyllidae (Palaearctic), and Ancistropsyllidae (Oriental), whereas families endemic to the southern continents (Australia, South America, Africa, Antarctica) include Malacopsyllidae and Rhopalopsyllidae (Neotropical), Stephanocircidae, Pygiopsyllidae, Stivaliidae, and Lycopsyllidae (Australian and Neotropical), and Xiphiopsyllidae and Chimaeropsyllidae (Afrotropical). The families Ctenophthalmidae, Ischnopsyllidae, and Pulicidae are more broadly distributed in both the Northern and the Southern Hemispheres.
CLASSIFICATION
The approximately 2575 species recognized in the order belong to 244 genera. The most recent review of the order by Lewis in 1998 recognizes 15 families, while Medvedev (1998) recognizes 18 families. Medvedev separated the pulicoid family into Pulicidae and Tungidae (long disputed by numerous workers), and fragmented the Pygiopsyllidae into three distinct families: Pygiopsyllidae, Lycopsyllidae, and Stivaliidae. The superfamilial relationships of these two classification schemes conflict in many respects and require modern DNA techniques to elucidate their true phylogenetic relationships.
PHYLOGENY AND EVOLUTION
Traditional phylogenetic hypotheses favored an origin of fleas from the Diptera or Mecoptera, but could not pin point the actual sister group. Current research suggests that the closest living relative to the fleas is a single mecopteran family, the snow fleas (Boreidae). This relationship is supported by the presence of unusual proven-tricular spines, a suite of ovariole characters (shared only by fleas and boreids), DNA sequence data, and other morphological and molecular characters (Whiting, 2001).
Phylogenetic relationships among Siphonaptera have been difficult to recover, in large part because of their extreme morphological specializations associated with ectoparasitism, which hampers the systematist from adequately homologizing characters across taxa. Though there have been attempts to reconstruct phylogeny across all flea groups based on morphology (Medvedev, 1994), these efforts have not relied upon modern phylogenetic methods and consequently lack a clear connection between character information and phylog-eny. A recent molecular phylogenetic analysis based on multiple loci for 100+ flea taxa provides a more comprehensive view of flea phy-logeny (Whiting et al., 2008). A summary tree is presented as Fig. 1 . From the superfamilial standpoint (Medvedev, 1994), these data support the monophyly of Pygiopsyllomorpha and Ceratophyllomorpha, but do not support Medvedev’s arrangement of the superfamilies Pulicomorpha and Hystrichopsyllomorpha. Of the 16 flea families examined in this analyses of Whiting et al., 10 are clearly monophyletic: Tungidae, Lycopsyllidae, Pygiopsyllidae, Stivaliidae, Stephanocircidae, Rhopalopsyllidae, Chimaeropsyllidae, Pulicidae, Ischnopsyllidae, and Ceratophyllidae. The families Leptopsyllidae, Hystrichopsyllidae, and Ctenophthalmidae are grossly paraphyletic. It is rather interesting that the neosomic Tungidae is placed as the most basal flea lineage and sister group to the remainder of the extant fleas. Overall, molecular data suggest that while the classification of fleas at the level of tribe and genus mostly reflect phylogeny, the higher classification of fleas is in need of serious revision to better reflect flea evolution.
MEDICAL AND VETERINARY SIGNIFICANCE
Fleas are capable of transmitting disease organisms to humans, including plague (Yersinia pestis) and murine typhus (Rickettsia typhi). They have also been implicated in the transmission of Q fever
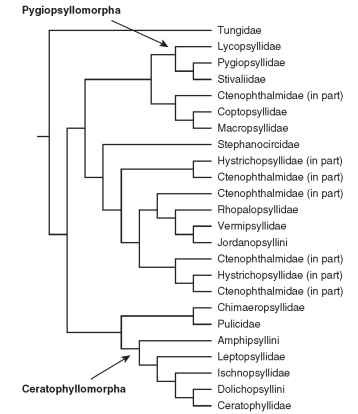
FIGURE 1 Phylogenetic tree of the Order Siphonaptera as determined by DNA analyses.
(Coxiella burnetti), tularemia (Franciscella tularensis), listeriosis (Listeria monocytogenes), salmonellosis, cat scratch fever (Bartonella henselae), and possibly Carrion’s disease (Bartonella bacilliformis). Several tapeworms occasionally infect humans who accidentally ingest fleas containing the intermediate stage (cysticercoid) of the tapeworms, including the double-pored dog tapeworm (Dipylidium caninum) and several tapeworms of rats (Hymenolepis diminuta, H. nana). Fleas also transmit a protozoan blood parasite (Trypanosoma lewisi) among rodents and a filarial worm (Dipetalonema reconditum) among dogs. Fleas are notorious for their biting and burrowing habits that cause intense itching and annoyance.
BIOLOGY
Detailed bionomic studies are available for only a small number of known flea taxa, so the following concepts are based on generalities found within the order.
Life Cycle
Fleas display four stadia (egg, larva, pupa, and adult) (Fig. 2A-D). The number and size of eggs and where they are laid differ from species to species, as does the time interval the larvae require to hatch. There are generally three instars. The exceptional neosomic Tunga monositus has only two larval stages. Larvae are ca. 1.5-10 mm in length, vermiform (worm-like), eyeless, legless, and without prominent features. Prior to pupation, the third instar ceases feeding and clears its gut contents by defecation. Silk glands present in this last instar are used to spin a cocoon prior to transformation into a quiescent exarate (appendages not “glued” to the body) pupa. The emergence of adults from the cocoon requires specific stimuli (e.g., temperature, vibrations) dependent on the adaptations of the individual species. Fleas may be univoltine or multivoltine. The optimum interval for development from egg to adult ranges from 3 weeks to more than a year. Adults may live for only a few weeks to more than 3 years.
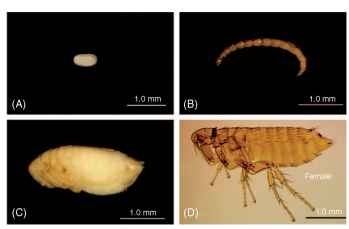
FIGURE 2 Life stages of a flea: (A) egg, (B) larva, (C) pupa, and (D) adult (a female is shown).
Reproduction
Males are smaller than females, have longer antennae, frequently have a dorsal groove on the occipital area of head, have less reticu-lar sculpturing on sternum II, and possess an elaborate aedeagus and modified terminal segments. The interantennal groove or falx frequently divides the preantennal and postantennal areas of the head in males, while this division is often absent in females of the same species. The details of copulation are understood in only a few species of fleas. In general, the male assumes a position under the female, clasps the ventral sternites of the female with his antennae (equipped with suction cup-line organelles), then clasps the female terminal segments (sternum VII) with the tergum IX (clasper), inserts the aedeagus, and transfers sperm by extrusion of long paired penis rods that penetrate the bursa copulatrix and duct of the spermatheca into the spermath-eca. Spermatozoa may be stored for long periods of time in the bulga (head) of the spermatheca. The hilla (tail) of the spermatheca of many species has an external muscle extended from its apex to the bulga. When an egg passes though the oviduct, the muscle contracts, pumping the sperm from the bulga through the duct of the spermatheca to the oviduct, where fertilization occurs through a micropyle in the surface of the egg. Depending on the species, the eggs may be laid on the host, in the nest, or randomly in the environment. The reproductive cycles of Cediopsylla and Spilopsyllus are linked to the hormonal estrous cycle of their rabbit hosts. Other species, such as Glaciopsyllus antarcticus, have their cycle synchronized with the seasonal migratory and nesting patterns of their avian hosts.
Host Specificity
Mammals harbor about 95% of the known flea species, with the remaining species living on birds. The seasonal and geographical distribution and host specificity of fleas are largely determined by specific requirements of the larvae. Each species has evolved a narrow range of tolerance for temperature and humidity, enabling exploitation of virtually every environment on the globe where avian or mammalian hosts flourish. These include the Antarctic (Glaciopsyllus) ; extreme xeric conditions of the world’s greatest deserts (Nosopsyllus, Hopkinsipsylla, Xenopsylla spp.); altitudinal extremes of the Andean, Himalayan, and Rocky mountains (Amphalius, Ctenophyllus, Geusibia, Ochotonobius, Craneopsylla, Plocopsylla, Cleopsylla, Stephanocircus) ; tropical rainforests (Polygenis, Rhopalopsyllus, Pygiopsyllidae); and coastal marine environments (Notiopsylla, Paraspsyllus). Although many species are catholic in their host preferences, the larval development is usually limited by the microenvironment of the individual host’s nest. Those demonstrating the least host specificity include those free-living taxa whose immature stages can tolerate wide ranges of temperature, humidity, and nutritional requirements (Ctenocephalides, Pulex).
Nutritional Requirements
Most flea species require a blood meal prior to oogenesis and spermatogenesis. With the exception of some species whose reproductive cycle is synchronized with that of their host, there are few studies on the nutritional requirements of adult fleas. Although the larvae of several fleas have been noted to burrow into dead host tissues, those of Uropsylla tasmanica are the only truly parasitic flea larvae that burrow into and feed on the tissues of their live hosts. Some larvae have been documented to feed directly on fresh blood as it is excreted from the anus of feeding adult fleas, but most scavenge on the dried blood residues or animal dandruff and excreta. A few species are predaceous on other small organisms within the nest.
Neosomy
Neosomy is the formation or enlargement of a morphological structure from secretion of new cuticle during an active stadium of a group that normally changes form by molting. Additional cuticle forms in neo-somes, accommodating expansion during enlargement (1000-fold) of gravid females that are capable of laying more than 1000 eggs. Neosomy occurs only among females because the male’s principal function is to mate with embedded females i n situ. Neosomatic growth occurs in the burrowing fleas Neotunga euloidea and all species of Tunga (Pulicidae). Fleas that do not become embedded, but attach for long periods of time, and also display neosomatic growth include members of Chaetopsylla, Dorcadia, Vermipsylla (Vermipsyllidae), Malacopsylla (Malacopsyllidae), and Hectopsylla (Pulicidae). Neosomatic fleas attach only once and do not feed repeatedly.
PRESERVATION OF FLEAS
Study of morphological structures with the aid of a compound microscope requires mounting of fleas on glass microscope slides. Traditional techniques include puncturing the area of abdominal sterna II and III with a minuten pin, soaking for 24 h in 10% potassium hydroxide, transferring to distilled water and gently compressing the flea’s abdomen to evaluate dissolved soft tissues, dehydrating in a series of ethanol solutions (70%, 80%, 95%, absolute) for 30 min each, clarifying the chitin for 15-20 min in methyl salicylate, transferring to xylene for a minimum of 1 h, and mounting in Canada balsam using a coverslip (No. 1 thickness). Fleas should be arranged with their heads to the right and their legs away from the technician. Thus, when viewed with a compound microscope, specimens will appear in an acceptable anatomical position with head to the left and legs toward the observer. Dissections are frequently required to permit observation of otherwise hidden structures.
MORPHOLOGICAL DIVERSITY AMONG FLEAS
For a general treatise of the morphology of adult fleas, refer to Hastriter and Whiting (2003), and the glossary of terms published in 1971 by Rothchild and Traub. As fleas co-evolved with a diverse number of avian/mammalian hosts, their morphology adapted accordingly and became equally diverse. This is well demonstrated in the cte-nidia (combs), spines, setae, and general morphology of fleas. These morphological entities have evolved to accommodate travel through a variety of host feathers and hairs. The lateral thickness of the head and shape of the frontal area (frons) also deviates greatly among the taxa. The head and thorax are used here to demonstrate the extreme morphological diversity among only four representative families of the 18 recognized families (Fig. 3A-L). The myriad of existing varieties in families not illustrated is beyond the scope of this treatise; however, their diversity of adaptive morphological features place this insect order among the most intriguing, ornate, and bizarre creatures in the insect world.
There have been several theories proposing the purpose and function of broad combs that occur in some flea genera. A most extreme example is that of Barreropsylla excelsa (Fig. 3F and 3L). Undoubtedly, combs afford some protection for the two adjoining segments. These may be present on the head and nota of the thorax and abdominal tergites. The protective armor and combs are best demonstrated in the combs and lateral plate of the pronotum of Corypsylla ornata (Fig. 3C and 3I ). Anomiopsyllus amphibolus (Fig. 3A and 3G) is an example of a flea with limited setae and no combs, or ornate vestiges. Since the flea’s main motility is through the feathers or fur of its host, the rearward directed combs prevent reverse travel and prevents the flea from being easily dislodged by a host during preening activities. It has even been suggested that these specialized combs have the ability to clasp hairs or barbules,
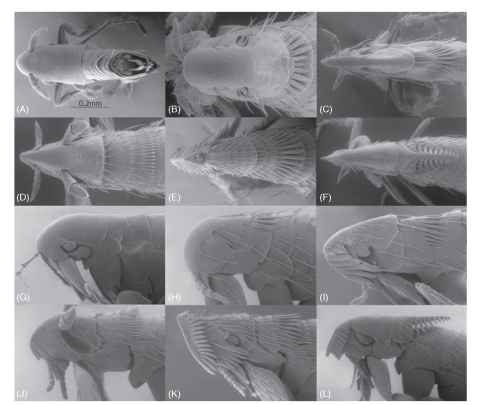
FIGURE 3 Dorsal view of head and thorax: (A) Anomiopsyllus amphibolus (Ctenophthalmidae), (B) Hoplopsyllus anomalus (Pulicidae), (C) Corypsylla ornata (Ctenophthalmidae), (D) Myodopsylla trisellis (Ischnopsyllidae), (E) Stephanocircus dasyuri (Stephanocircidae) (F) Barreropsylla excelsa (Stephaocircidae). Lateral view of head and thorax: (G) Anomiopsyllus amphibolus (Ctenophthalmidae), (H) Hoplopsyllus anomalus (Pulicidae), (I) Corypsylla ornata (Ctenophthalmidae), (J) Myodopsylla trisellis (Ischnopsyllidae), (K) Stephanocircus dasyuri (Stephanocircidae), (L) Barreropsylla excelsa (Sephanocircidae).
although this theory has never been proven. The wedging effect of combs arranged in parallel may have some advantage to affix the flea in place when pulled back through the fur or feathers by a preening host, or when the flea attempts to feed, head combs may provide some advantage in wedging the head against the host skin while the flea pierces the host during feeding. The latter is noteworthy in bat fleas (Ischnopsyllidae) that have two spatulate combs at the oral angle of the head (Fig. 3D and 3J) and Stephanocircus dasy-uri (Stephanocircidae) that have elaborate combs on a frontal head plate that overlaps the separate main portion of the head (Fig. 3E and 3K). Combs may be spatulate, while others are sharply pointed (Fig. 3D and 3E ) . In general, fleas found on avian hosts tend to have greater numbers of setae, and the setae are longer and thinner than those found on mammalian hosts. The shape of the head also varies greatly as the anterior and dorsal portions of the head of some fleas are sharply “conical” and thinly streamlined (Fig. 3C-F), while that of others are broad and blunt (Fig. 3A-B). Although quite unrelated, other ectoparasites [Streblidae, Nycterobiidae (Diptera), Polyctenidae (Hemiptera), and Leiodidae (Coleoptera)] have independently evolved very similar and convergent patterns of combs and other vestitures as that of those found in Siphonaptera.
ANATOMY OF LARVAE
The diversity of morphological characters parallels those of adult fleas. Important structures include the shape of the egg tooth (first instars only); chaetotaxy and sensory structures of the head capsule,antenna, and antennal mound; cuticular sensilla of the dorsal plates of the thorax and abdomen; number and length of body setae; sculpturing of the integument; and arrangement of the anal comb and other setae on the 10th abdominal segment.
CONTROL OF FLEAS
Control is often necessary when fleas parasitize livestock and pets, become potential vectors of disease, or are biting people. Household sanitation such as thorough vacuuming of indoor areas and washing of pet bedding removes many of the immature stages (eggs, larvae, and pupae). Insect growth regulators (methoprene and pyriproxyfen) that inhibit the development of immature stages are used in conjunction with organophosphates (chlorpyrifos and diazinon) to interrupt the developmental cycle. Dog and cat flea collars and the use of systemic insecticides add additional preventive measures. Control of potential plague vectors in rural or sylvatic environments requires more drastic measures. Wild mammals such as rats, prairie dogs, ground squirrels, and chipmunks must be controlled around human habitations and recreation sites frequented by people. In such situations, it is necessary to apply Carbamates (carbaryl) to burrows and rodent harborages prior to control of animal populations. Failure to control fleas prior to implementing animal control endangers humans that may come into contact with these potentially disease-infected fleas.
