Silk Production
Silks are ectodermal, proteinaceous secretions that polymerize into solid fibers in the external environment. Silk proteins might have evolved from cuticular secretions and occur in all three subphyla of terrestrial arthropods. However, only the silks produced by a few insects and some spiders have been examined in detail. The silks of a dozen lepidopteran species are used commercially for the production of fabrics and to a small extent in the cosmetics and medicine.
THE MULTIPLE EVOLUTIONARY PATHWAYS OF SILK PRODUCTION IN INSECTS
Phylogenetic comparison of the silk producing hexapods suggests that silk production evolved multiple times, often only in larvae or adults, rarely in both stages (Figs. 1 and 2 ). In the apterygote hexapods, silk is used to aid the sperm transfer to females and some adults use silk to protect their eggs. Silk production is rare in the hemimetabolous clades, and common in Holometabola, in which it is most often used to protect the pupa. All known silk glands are of ectodermal cell linage but specialized dermal glands, which are
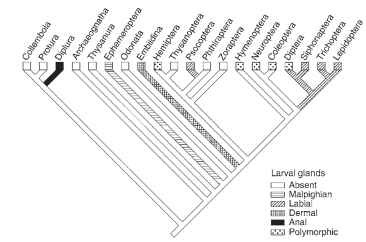
FIGURE 1 Phylogenetic analysis of silk production by hexapod larvae. The most prolific silk producers, such as Embiidina, Psocoptera, and several orders of Holometabola, have evolved dedicated silk glands.
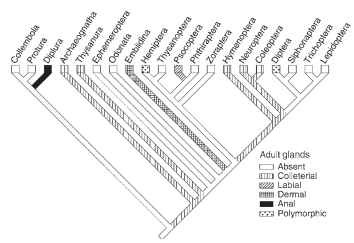
FIGURE 2 Silk production by hexapod adults is less common than among larvae. Most frequently, adult insects produce fibrous proteins in colleterial glands.
common in spiders, are found rarely in the insects. Insect silks are typically produced by modified parts of the digestive, excretory, and reproductive systems, respectively. In a number of cases the source of silk has not been identified with certainty.
Dermal glands dedicated to silk production evolved in the frontal tarsi of Embiidina which spend most of their lives in silky tunnels. Tarsal glands function both in the larvae and adults, particularly in the females, which employ silk to protect their eggs and youngs. Similar tarsal glands occur also in adult males of the Hilarinae fly family; silk is used to package prey given to the female during courting. Dermal glands also seem to occur in the cerci of adult Archaeognatha and Thysanura. The abdominal silk glands of Protura and possibly also Diplura may represent a kind of dermal glands.
The secretions of modified salivary glands, and some of the anal excretions, represent silks derived from the digestive system. Some larval and adult Gryllacridoidea (Orthoptera) produce silk in labial glands and use it to “glue” leaves, stones, and soils into protective covers of ground burrows. Silk production in the labial glands occurs in the large larvae and especially in the adults of Psocoptera and in the larvae of Hymenoptera, Trichoptera, Lepidoptera, Siphonaptera, and several families of nematocerous Diptera. The families of all these orders differ in the extent of silk production. For example, among the Psocoptera, the Psocidae cover their eggs with silks, the Elipsocidae and Philotarsidae spin webs singly or gregariously in small groups, and the Archipsocidae spin extensive webs covering trunks and branches of large trees.
Cocoon spinning at the end of larval development is the most common silk use in the Holometabola. The larvae of some Trichoptera employ silk to construct protective cases (they also occur in a few lepidopteran families) or nets for catching prey (Fig. 3) , and some caterpillars spin large webs in which they hide (Fig. 4) . The larvae of water midges (Chironomidae) live in individual silk tubes. Larvae of the nematoceran family Mycetophilidae catch prey with the aid of sticky silk threads. Larvae of several beetle families construct cocoons from anal excretions that are refered to as peritrophic membrane but they could represent proctodeal cuticle. Proctodeal epithelium was showed to be the source protein glues added to the surface of silk fibers produced in the Malpighian tubules in larval Neuroptera. The glue-like or silk-like anal excretions in adult Thysanoptera may also be derived from the proctodeum.

FIGURE 3 Catching net of the caddisfly Hydropsyche angusti-penni. The larva hides in a tube that opens at the edge of the net.

FIGURE 4 Caterpillars of the ermine moth Yponomeuta cagnag-ella in their communal hiding web. The web with scattered excrements functions as a camouflage for the caterpillars that are light with black spots.
Silk production in the Malpighian tubules of larvae evolved in a few Ephemeroptera and Coleoptera, and is apparently plesiomorphic in Neuroptera. The rare silk of mayfly larvae is probably used as walking substrate, whereas in the coleopteran and neuropteran larvae it is used to construct cocoons. Larvae of the phylogenetically primitive neurop-teroid taxa, Megaloptera and Raphidioidea, are also said to produce silks in Malpighian tubules, and the larvae of some Hymenoptera, for example of the stingless bee Plebeia droryana and the bumble bee Bombus atratus, bind silk fibers derived from the labial glands into the cocoon wall with the Malpighian tubule secretions.
At least five taxa of adult insects produce silks in the colleterial glands of the reproductive system. The males of Thysanura (the bris-tletails) produce protein secretions in the accessory glands and release it through a specialized spinning organ. Female mantids (Mantoidea) produce silk in the colleterial glands to protect their eggs, as do beetles in the family Hydrophilidae and the lacewings (Neuroptera). Neuroptera lay their eggs on silk stalks thereby protecting them from predatory mites and bugs crawling on the leaf surface.
THE COMPOSITION OF SILK
Labial silk glands of all holometabolous larvae are composed of a relatively small number of highly polyploid cells. The glands of caterpillars are differentiated into a posterior, middle, and anterior sections. The posterior section secretes a “heavy chain” fibroin (>200kDa) that is in most species associated with two axilliary proteins (25-31 kDa) called “light chain” fibroin and P25. The heavy chain fibroin is composed of species-specific repetitive regions that are built of simple motifs. The repeated regions, in part, determine the molecular conformation of fibroin, hence the mode of fiber formation. Stacked (3-pleated sheets were detected in the preparations of different silks and are often regarded as the standard conformation of lepidopteran fibroin. However, X-ray crystallography and alternative fibroin sequences indicate more complex protein configurations in the silk of some species.
A highly concentrated protein gel produced in the posterior section is pushed into the middle silk gland section where it is enveloped by the sericin proteins. Sericin secretion occurs along the middle section according to a precise spatial and temporal pattern that determines which sericins are adjacent to the fibroin column and which are exterior. The shearing forces and dehydration of the silk dope during its passage through the anterior silk gland section cause fibroin solidification into a filament. Two filaments, one from the left and one from the right labial gland, are aligned and sealed together into a fiber by inner sericins when they pass through the common spinneret. Outer sericins remain sticky for some time after leaving the spinneret and glue consecutive fiber layers into the cocoon wall.
The term “fibroin” has been adopted for any thread-like silk protein but true silk gene homology with Lepidoptera probably applies only to their sister order Trichoptera. The silk of caddisflies contains heavy chain fibroin, light chain fibroin, sericin-like proteins, and additional, cysteine-rich proteins not found in the lepidopteran silk. The nature of repeat sequences in the fibroins of Trichoptera is distinct from the silks produced by the Lepidoptera.
The silks produced by the water midges (Chironomidae: Diptera) contain more than 15 mutually homologous proteins that fall into three size categories (from <100 to about 1000 kDa). They consist of repeats that seem unrelated to any lepidopteran silk. The composition of silk produced by fleas (Siphonaptera) is apparently unique by having high content of the glutamic acid and a-helical conformation. The few silk proteins identified in Hymenoptera are also unrelated to those of Lepidoptera. The sawflies spin silks based on the 3 -sheets, collagen-like proteins, or on the polyglycine II proteins, and the silks of analyzed Apocrita are either 3 -sheet structured or a-helical coiled coil.
The sequence, configuration, and composition of silks derived from glands other than the labial glands have been very less investigated. The only unifying feature of the silks from different insect orders is the repetitive nature of the major protein(s). Convergent evolution driven by the need of certain physical silk properties, minimal energy investment, and the scarcity of the essential amino acid yields repeated sequences rich in glycine, alanine, and ser-ine. Similar conformations of the silk proteins are also products of convergent evolution. For example, silk derived from the larval Malpighian tubules in the beetle Hypera postica appears as a loosely fabricated networks of coarse brown fibers composed of proteins in cross-3 conformation. Colleterial gland silks were also reported to have either a cross-3 or a-helical configuration. All of the parallel-3 silks are pulled (spun), in contrast to the a-helical and cross-3 silks, which are deposited, secreted, or ejected. Silks produced in aerial environment apparently polymerize into fiber by weak molecular interactions but silk polymerization and stability under water in the larvae of caddisflies and chironomids may rest on the covalent bonds in the disulfide bridges.
HUMAN USES OF SILKS
Practical utilization of silk was discovered in China about 2700 years B.C. The domesticated silkworm, Bombyx mori, and a few wild-living lepidopterans (mostly from the family Saturniidae) provide cocoons suitable for harvesting of silk fiber. To produce yarns, the cocoons are soaked or boiled in slightly alkaline water for several minutes to a few hours. The outer sericin layer is dissolved and the loosened fiber is pulled out and aligned (“spun”) with several other fibers into a thread that can be used to produce textiles. The sericulture industry declined with the invention of nylon and other synthetic fibers. Nevertheless, natural silk remains a source of materials for luxury textiles, cosmetics, food additives and in multiple medical applications. Small-scale, wild silk production targeted to border forests surrounding protected areas is being used as a tool in conservation studies to interest farmers in maintaining native trees and shrubs.
