The primary goals of the insect respiratory system are to deliver oxygen from the air to the tissues and to transport carbon dioxide from the tissues to air. In contrast to many other animals, most oxygen and carbon dioxide transport occurs in the gas phase, with gases transported through the tracheal system by both diffusion and convection. However, insects do have oxygen-binding pigments that likely assist in gas exchange, with primitive insects having hemocyanin in the hemolymph and probably all insects possessing hemoglobin in certain tissues. The structures and physiological mechanisms of the respiratory system vary dramatically with phylogeny, developmental stage, and habitat. With a given tracheal structure, the ability of the tracheal system to transport gases can be modulated dramatically by varying spiracular opening, ventilation, and the fluid level in the tracheoles.
MECHANISMS OF GAS EXCHANGE
Insect gas exchange occurs in a series of steps. Oxygen molecules first enter the insect via the spiracle, then proceed down the branching tracheae to the tracheoles. The terminal tips of the tracheoles are sometimes fluid-filled, so at this point gas transport may occur in a liquid medium rather than air. Oxygen then must move across the tracheolar walls, through the hemolymph, across the plasma membranes of the cells, and finally through the cytoplasm to the mitochondria. Carbon dioxide generally follows a reverse path.
Diffusion
Diffusion is the passive movement of molecules down their concentration gradient, driven by random molecular motions. Because oxygen is transported to the tissues as a gas and the diffusion rate of oxygen is much more rapid in air than in water, the insect tra-cheal system is capable of high rates of gas exchange by diffusion. Consumption of oxygen by the tissues lowers internal oxygen levels, creating a partial pressure gradient from air to tissues that drives diffusion of oxygen through the tracheae. The converse occurs for carbon dioxide. The final steps of oxygen delivery, from the tracheoles to the mitochondria, may occur by diffusion in all insects, because diffusion operates rapidly over micron distances. In the initial steps of oxygen delivery (across the spiracles, through the tracheae), the importance of diffusion is more variable. Simple diffusive gas exchange through the tracheae and spiracles likely occurs in some pupae, as washout rates of inert gases are similar to those predicted from their diffusion coefficients. Diffusion is clearly the only mechanism of gas exchange in insect eggs, with the major layer providing resistance being either the crystalline chorionic layer or the wax layer of the egg shell. Unlike vertebrates, most insects can recover from exposure to anoxia; during such recovery oxygen delivery to the mitochondria seems very likely to occur by diffusion since the ventilatory muscles are paralyzed.
Convection
Convection is the bulk movement of a fluid (gas or liquid) driven by pressure. Differential air pressures can drive gas movement through the tracheae and spiracles at much higher rates and over longer distances than diffusion. Tracheal and air sac collapse and inflation can now be observed within living insects using modern imaging systems such as phase-contrast synchrotron imaging (Fig. 1 ).
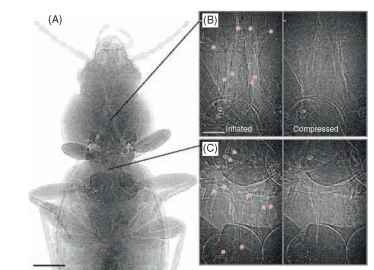
FIGURE 1 Phase contrast X-ray synchrotron images of a carabid beetle. (A) Cranial half of animal, tracheae appear as light-colored tubes, scale bar: 1 mm. (B) and (C) Inflated (left) and deflated (right) tracheae in the mesothorax (B) and metathorax (C), red stars indicate tracheae, scale bar 200 |im; the frequency of these cycles is 10-15 min-1
The advantages that insects gain by using convective gas exchange are somewhat controversial. In general, the use of convective gas exchange increases with metabolic rate, suggesting that convection is necessary to allow most insects to achieve high rates of gas exchange. However, it is also plausible that the increased convection is at least also important for other functions such as reducing gradients for oxygen within the active insect or is a byproduct of hemolymph pumping.
The mechanisms by which insects achieve convective gas exchange are complex and varied. In many insects, well-coordinated actions of muscles and spiracles produce regulated convective air flow through the tracheae and spiracles. Most commonly, convection is driven by contractions of respiratory muscles attached to the body wall, which produce increases or decreases in body volume, causing compressible portions of the tracheal system to inflate or deflate.
One common mechanism by which insects accomplish convec-tive air flow through the trachea is abdominal pumping. Expiratory muscles connect the ventral and dorsal cuticular plates and also span adjacent abdominal segments. When they contract, they pull the dorsal terga and ventral sterna together (Fig. 2) and the tip of the abdomen inward as the cuticular plates slide over the flexible pleural and intersegmental membranes. When the body volume decreases,
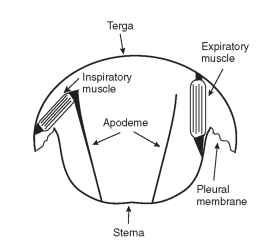
FIGURE 2 Respiratory muscles that drive the dorso-ventral movements of the abdomen during abdominal pumping in a grasshopper.
the cuticle pushes on hemolymph and tissues, which in turn compress the collapsible air sacs. Inspiration may be passive and result from cuticular elasticity. Alternatively, contractions of inspiratory muscles attached to tall sternal apodomes and the lower edge of the terga can lift the terga relative to the sterna and expand abdominal volume and the air sacs (Fig. 2). Abdominal pumping drives hemolymph pulsations and tracheal air flow in a wide variety of adult, larval, and pupal insects.
In many of the insects that have been examined, abdominal pumping is coordinated with spiracular opening in a manner that produces directed flow through particular spiracles. In some insects, unidirectional air flow through the longitudinal tracheal trunks occurs. During inspiration, abdominal spiracles are closed and air flows in through open thoracic spiracles (Fig. 3). During expiration, air flows out open abdominal spiracles, while the thoracic spiracles are closed. There is evidence for unidirectional air flow in adult Dictyoptera, Orthoptera, Hymenoptera, Coleoptera, and Odonata. In some pupae, abdominal pumping is coordinated with the opening of one or a few ” master spiracles ” that exchange all gases.
Muscles that have the primary purpose of driving hemolymph circulation, such as the heart and ventral diaphragm, also play a role in creating convective ventilation. Pumping of the heart and accessory muscles
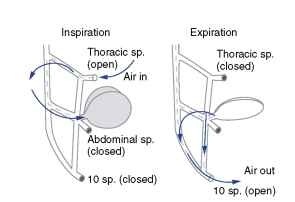
FIGURE 3 Unidirectional airflow during abdominal pumping in a grasshopper. During inspiration, air flows in through open thoracic spiracles (sp), along the longitudinal trachea, and into the air sacs. At low metabolic rates, air flows out only through the tenth abdominal spiracles; in more active animals, air flows out all abdominal spiracles.
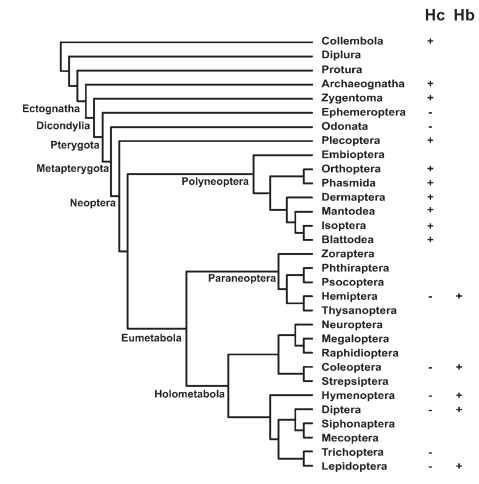
FIGURE 4 Distribution of respiratory proteins in insect order. Hc = hemocyanin;Hb = hemoglobin.
at the base of the wings, antennae, and legs pushes hemolymph and air into these appendages. In a variety of adult Lepidoptera, Diptera, and Hymenoptera, the heart occasionally reverses pumping direction, shifting hemolymph from the thorax to the abdomen. As the hemolymph accumulates in one body compartment, it compresses air sacs in that compartment, causing convective air flow through tracheae and spiracles.
OXYGEN-BINDING PROTEINS AND OXYGEN SENSING
Oxygen-binding pigments such as hemoglobin have been thought to be unimportant in gas exchange for most insects, due to the high capacity of tracheal systems. However, it is becoming apparent that oxygen-binding pigments are very important for gas exchange in a variety of insects. Both hemoglobin and hemocyanin play roles in insect gas exchange, with their distribution being at least partly related to phylogenetic history (Fig. 4 ).
Hemocyanin, which is the respiratory protein found in the crustacean ancestors of insects, occurs in the hemolymph of a wide variety of primitive insect orders including Collembola, Zygentoma, Plecoptera, Orthoptera, Isoptera, and Blattoidea, but not in the Eumetabola. At least in one plecopteran, hemocyanin occurs at relatively high concentrations in the hemolymph, and the oxygen affinity of the hemocyanin is consistent with a role for this protein in oxygen delivery, suggesting that the hemolymph hemocyanin may play a significant role in oxgyen delivery for a variety of insects.
Hemoglobin only occurs in the hemolymph of aquatic larval and pupal chironomids. These insects possess high-affinity hemoglobins that help them extract and store oxygen from hypoxic environments. Certain other aquatic insects have specialized tissues that contain high concentrations of intracellular hemoglobin that extract and store oxygen. One example is the larvae of the botfly, Gastrophilus intestinalis, which parasitizes horse intestines and obtains oxygen from intermittently available bubbles swallowed by the host. Backswimmers (Hemiptera) have specialized organs, penetrated by tracheoles, that contain hemoglobin. When backswimmers dive, oxygen is transferred from the air bubble they carry to the hemoglobin, stabilizing their buoyancy, and prolonging their dive duration.
Intracellular hemoglobins have also recently been detected in the tracheoles and a variety of other tissues of a wide variety of insects including the fruit fly, Drosophila melanogaster, the honey bee, Apis mellifera, and the mosquitoes, Aedes aegypti and Anopheles gambiae. The concentration of hemoglobin in these tissues is unknown. These hemoglobins are evolutionarily similar to crustacean hemoglobins, suggesting that intracellular hemoglobins are common and primitive in insects. The functions of these hemoglobins are unknown and may include oxygen storage for use during hypoxia or burst performance, enhancement of oxygen transport within tissues, detoxification of reactive oxygen species, and oxygen signaling.
Oxygen-sensing proteins allow insects and other animals to monitor internal oxygen levels and respond appropriately. One major class of oxygen sensors are the hypoxia-inducible factors (HIF), which serve as transcription factors. The HIF signaling pathway is conserved from worms to humans, generally serving to increase oxygen delivery capacity and anaerobic capacities in response to hypoxia. The abundance and activity of HIF protein is inversely related to tissue oxygen levels in Drosophila, due to oxygen effects on HIF oxidation by prolyl hydroxylase (and subsequent degredation of HIF protein). Relatively few studies have yet examined the functional consequences of increased HIF levels in response to hypoxia, but there is evidence suggesting that increases in HIF may trigger tracheal proliferation and reduced cell (and body) size via effects on the cell cycle.
A second protein implicated in responses to hypoxia are the atypical guanyl cyclases. The product of guanyl cyclase, cyclic GMP (cGMP) is an important intracellular second messenger for many processes. Atypical guanyl cyclases are activated by hypoxia, and in Drosophila, cGMP levels are inversely related to oxygen partial pressures. There is evidence that increasing cGMP may mediate the rapid escape response observed when fruit fly larvae are exposed to hypoxia.
DISCONTINUOUS GAS EXCHANGE
For many insects, especially those that are highly active, the spiracles close for only brief periods if at all, and oxygen and carbon dioxide are exchanged relatively continuously. However, many insects exhibit discontinuous gas exchange. During discontinuous gas exchange, periods of spiracular closure (in which no or reduced gas exchange occurs) alternate with periods of spiracular opening (and greatly increased gas exchange).
Discontinuous gas exchange has been most extensively studied in ants and diapausing lepidopteran pupae. In lepidopteran pupae, spiracular openings can be separated by hours. After the burst of gas exchange, the spiracles hermetically seal (closed phase). While the spiracles are closed, oxygen is consumed from within the tracheal system. The oxygen removed from the tracheal air space is not completely replaced by carbon dioxide, primarily because a large fraction of the carbon dioxide produced dissolves in the tissues and hemolymph (carbon dioxide, in contrast to oxygen, is highly soluble in biological fluids). Therefore, pressures fall below atmospheric within the tracheal system and abdominal length is reduced. When internal oxygen tensions reach a low threshold, the spiracles begin to open slightly at high frequency (flutter phase), and tracheal pressures rise to near-atmospheric levels. During the flutter phase, high-frequency, but minutely subatmos-pheric, air pressures allow the animal to convectively take in oxygen with minimal emission of carbon dioxide. The spiracles remain sufficiently closed so that internal oxygen levels remain low. Carbon dioxide accumulates throughout the closed and fluttering phases, eventually triggering the spiracular open phase, when tracheal oxygen levels are restored to near-atmospheric partial pressures.
Discontinuous gas exchange patterns and mechanisms are quite variable among and within species. Many insects only exhibit discontinuous gas exchange when at rest or during diapause. However, some highly active insects, such as running ants, also exchange gases discon-tinuously. When metabolic rates increase (at higher temperatures or during activity), the time period between spiracular phases tends to decrease. Significant carbon dioxide is lost during the flutter phase of many insects, suggesting that outward diffusive gas exchange occurs during flutter phase of these species. Another variant is the use of abdominal pumping to enhance gas exchange during the open phase.
The functional significance of discontinuous gas exchange remains controversial. A variety of cross-species comparisons suggest that insects evolved to live in warmer or more arid regions have longer and more pronounced discontinuous gas exchange cycles. However, within-species experiments have often indicated that switching in and
out of discontinuous gas exchange has little effect on water loss for the insects examined. Other current, nonalternative hypotheses are that discontinuous gas exchange: (1) protects tissues from reactive oxygen species by keeping internal oxygen levels low, (2) enhances gas exchange during hypoxia, and (3) is simply a consequence of having spiracles controlled in a relatively binary fashion (open vs. closed).
AQUATIC AND ENDOPARASITIC INSECTS
Insects can obtain oxygen while living in fluid environments and are common in the shallows of fresh waters, brackish estuaries, and as endoparasites. Aquatic and endoparasitic insects generally retain an internal, air-filled tracheal system. The air-filled, buoyant tracheal system may inhibit insects from being able to dive deeply and avoid predators, limiting their ecological success in deep waters.
Many aquatic and endoparasitic species have anatomical features that allow them to feed underwater (or within the host) while maintaining contact with air. For example, in many aquatic dipteran larvae such as mosquitoes, the posterior spiracles are surrounded by water-repellent hairs and are kept in the air while the animals feed in a head-down position. In water scorpions (Hemiptera: Nepidae), spiracles are located on the end of a long tube which is extended up to the water surface. Similarly, endoparasitic insects such as chalcid (Hymenoptera) larvae and tachinid (Diptera) larvae connect to the air using posterior spiracles inserted through the host’s integument or tracheal system.
Some Coleoptera and Heteroptera use their hairs or wings to carry air bubbles adjacent to their spiracles when they dive. These air bubbles serve as oxygen stores and as temporary gas exchange structures. Oxygen is removed from the air bubble by the diving insect, causing oxygen partial pressures in the air bubble to fall below that in the surrounding water. Oxygen consumption from the bubble also causes the nitrogen partial pressure in the air bubble to rise above that in the surrounding water, so that nitrogen leaves the bubble to the water by diffusion. Eventually, when all the nitrogen leaves, the bubble disappears and the insect must return to the surface.
Many aquatic and endoparasitic insects never access air and must obtain oxygen directly from water or the blood of the host. Some aquatic insects that rarely visit the surface obtain oxygen from the water by having specialized structures called plastrons which hold a thin film of air on the outside of their body. These insects have a thick (as many as 2 million hairs per mm2) layer of short water-repellent hairs that resist wetting and do not collapse under pressure. Spiracles open directly into the air space of the plastron. Plastrons are thought to behave as gas exchange structures in a manner similar to air bubbles. However, because the dense hairs make the plastron incompressible, oxygen removal from the plastron by the insect will lower both the total pressure and the oxygen partial pressure in the air of the plastron. Thus nitrogen levels are likely to remain near to those in water, and the air and gas exchange function of the plastron can persist indefinitely.
Many aquatic or endoparasitic insects that do not access air lack spiracles, and so oxygen must be transported by diffusion from the water, across the cuticle, and then into the tracheae. A common feature of these insects are tracheal gills, leaflike structures of thin cuticle containing many tracheae and tracheoles, that increase the available surface area for obtaining oxygen from water (Fig. 5). Gills may be on the abdominal tip (Diptera, Odonata: Zygoptera), laterally along the abdomen (Ephemeroptera, Trichoptera), or within the rectum (Diptera, Odonata: Anisoptera).

FIGURE 5 Caudal lamellae, which function as tracheal gills.
As oxygen is absorbed across a gill, oxygen level in the boundary layer of water over the gill can become low, slowing diffusive influx of oxygen. To overcome this problem, muscles drive rhythmic waving of abdominal gills, forcing fresh, oxygenated water to flow over the gill, thereby reducing boundary layer thickness. For similar reasons, rectal gills are ventilated by alternate contractions and relaxations of the rectum, which drive water in and out of the anus. Such ventilatory movements are enhanced in water containing low levels of oxygen.
PLASTICITY IN TRACHEAL STRUCTURE AND FUNCTION
An insect’s need for oxygen and production of carbon dioxide varies tremendously, as metabolic rate varies strongly with transitions from rest to flight, unfed to fed state, or from diapause to growth. When metabolic rates increase, there is an increased need for oxygen uptake and carbon dioxide removal. Insects also require a higher capacity to exchange gases when they are exposed to low-oxygen conditions such as hypoxic waters or high altitudes.
In the short-term, increased gas exchange with a set tracheal system structure can be accomplished by at least four nonexclusive mechanisms. First, insects can simply tolerate lower internal oxygen and higher internal carbon dioxide partial pressures. The increased gas partial pressure gradients will enhance gas exchange by either diffusion or convection. Secondly, insects can increase diffusive gas exchange by opening the spiracles to a greater degree or for longer time periods. Third, insects can increase convective ventilation through the spiracles and tracheal system (terrestrial insects) or over the gills (aquatic insects) by mechanisms such as abdominal pumping or gill waving. Convection in the hemolymph may also be important for insects that utilize hemolymph hemocyanin. Fourth, insects can reduce fluid levels in the tracheoles, enhancing diffusive oxygen delivery within the tissues because of the faster diffusion of oxygen through air than water. In the longer term, alterations in tracheal morphology (e.g., tracheal diameter and number of tracheoles) and perhaps the level of oxygen-binding pigments may affect gas exchange capacity.
Locomotion
Locomotion requires muscular activity and increased ATP turnover, which increases the organism’s need for oxygen. Running insects increase their gas exchange rates by 2-10 fold relative to resting conditions. Flight is usually associated with larger increases in gas exchange, especially in insects that maintain thoracic temperatures near 40°C, such as bees and dragonflies. These insect athletes have oxygen consumption rates among the highest known in the animal kingdom, reaching levels 100 times higher than those of cold, resting insects.
Relatively little is known of the mechanisms by which insects increase gas exchange during terrestrial locomotion. In a cockroach, and a grasshopper, internal carbon dioxide levels rise while oxygen levels fall during running and hopping, respectively. In the grasshopper Melanoplus bivittatus, abdominal pumping rates are low during hopping, but increase relative to resting rates afterward, increasing convection and restoring tracheal gases to normal, resting levels. Even though there is no evidence for increased abdominal pumping during hopping, increased convective ventilation may occur due to pressure fluctuations associated with cuticular deformations associated with jumping.
During flight, convective gas exchange can be increased by thoracic autoventilation and abdominal pumping. Thoracic autoventilation occurs when flight muscle contractions cause compression of the air sacs within the thorax, producing strong convective ventilation. Such increases in thoracic autoventilation have been shown for grasshoppers, ceramby-cid, elaterid, and anthribid beetles, moths (Lepidoptera), and dragon-flies (Odonata). Another method for increasing gas exchange has been shown for the gigantic cerambycid beetle (Petrognatha gigas) in which wind pressure generated by forward flight drives convective air flow through the major tracheal trunks. Abdominal pumping is considered the major mechanism by which convective ventilation is increased during flight in large Hymenoptera, Diptera, and scarabaeid and buprestid beetles, and abdominal pumping supplements autoventilation during flight in dragonflies and grasshoppers. In the insects studied to date (a moth and a bumblebee), oxygen partial pressures in active flight muscles are maintained at levels similar to those in resting animals, suggesting that increases in the capacity of the tracheal system to conduct gases match increased tissue needs for oxygen during flight.
During Hypoxia
Insects may encounter low environmental oxygen availability in a number of locations including hypoxic waters, burrows, feeding sites within large dense structures such as granaries, or at high altitude. Terrestrial insects are generally quite good at coping with hypoxia and, usually, can maintain resting metabolic rates down to atmospheric oxygen levels of 1-5 kilopascals (normal atmospheric oxygen partial pressure is 21 kilopascals). The large safety margin for oxygen delivery of resting terrestrial insects probably reflects the fact that tracheal systems must be designed to allow the much higher rates of gas exchange during activity. In support of this hypothesis, metabolic rates during flight are generally more sensitive to hypoxia, with flight metabolism being inhibited at 8 kilopascals in hovering honey bees, at 10 kilopascals in tethered flying flies, and being stimulated by hyperoxia in a dragonfly. Growth rates are inhibited by relatively mild hypoxia (10 kilopascal), at least in larval mealworms (Coleoptera) and fruit flies (Diptera), suggesting that such moderate hypoxia limits some physiological processes even in nonlocomoting insects.
Insects respond to hypoxia by increasing tracheal gas exchange capacity. In a wide variety of insects, hypoxia induces spiracular opening. In ants and lepidopteran pupae exhibiting discontinuous ventilation, exposure to hypoxia increases the frequency and duration of spiracular opening. In adult grasshoppers, exposure to hypoxia causes a strong increase in convective ventilation, mostly accomplished by an increase in the frequency of abdominal pumping. Aquatic insects with gills generally increase convective water flow past the gills in response to hypoxia, either by increasing the frequency of their gill-beating (Ephemeroptera), body undulations (Plecoptera), or rectal pumping (Odonata). Aquatic insects exposed to hypoxia often move to faster flowing water, to the water surface, or even into the air.
Insects can exhibit changes in tracheal system structure in response to longer-term exposure to hypoxia. For example, the transverse tracheae of mealworms (Coleoptera) and fruit flies (Diptera) increase in size when reared under conditions of low oxygen availability. Tracheole growth and branching are stimulated by hypoxia in the epidermis of Rhodnius (Hemiptera) and fruit flies. Similarly, in some larval Ephemeroptera, gill area is inversely proportional to environmental oxygen levels.
CONTROL OF RESPIRATORY FUNCTION
The spiracular muscles are primarily controlled by nerves from the central nervous system. The motor neurons to the spiracular muscles arise from ganglia in the same segment or that immediately anterior. In dragonflies, Periplaneta cockroaches, and Schistocerca grasshoppers, the spiracular closer muscles are innervated by two motor nerves that branch from the median nerve sending an axon to each side of the animal, so that both spiracles on a segment receive similar neural input. In the prothoracic spiracle of Schistocerca, two motor nerves innervate the opener muscle from the prothoracic ganglia and one motor nerve arrives from the mesothoracic ganglia. Increasing action potential frequencies in these nerves stimulate increasing muscle activity, resulting in gradations in the magnitude and duration of spiracular closing.
Current information suggests that for spiracles with two muscles, effects of carbon dioxide or oxygen are mediated centrally. When high levels of carbon dioxide or low levels of oxygen are applied to the ventral ganglia or head, the frequency of action potentials to the closer muscles decreases and the frequency of action potentials to the opener muscles increases, increasing spiracular opening. Temperature increases or flight also affects the frequency of action potentials to these spiracular muscles.
In contrast, data for spiracles with only a closer muscle suggest that carbon dioxide acts peripherally, while oxygen acts centrally. Central application of hypoxic gases stimulates a fall in the action potential frequencies to the closer muscle, whereas carbon dioxide has little effect. However, when carbon dioxide is applied directly on the spiracular muscle, there is a fall in the muscle membrane depolarization resulting from nerve stimulation, a decrease in closer muscle tension, and increased spiracular opening. Spiracle opening is stimulated by a rise in carbon dioxide but not a decrease in extracellular pH, suggesting that the carbon dioxide effect is not mediated by pH changes.
The rhythmic abdominal pumping movements that drive convec-tive ventilation in many insects are initiated by central pattern generators in the metathoracic ganglia or the first abdominal ganglia. These rhythmic ventilatory bursts can be demonstrated in nerve cords isolated in vitro. The motor output from these central pattern generators passes down the ventral nerve cord via interneurons and stimulates rhythmical sequences of respiratory muscle activity.
In grasshoppers (Orthoptera) and probably many other insects, the rate of abdominal pumping can be altered by sensory output from stretch receptors in the abdomen, chemosensors located in the thorax and head, and by feed-forward control. Increasing carbon dioxide or decreasing oxygen partial pressures in the tracheae stimulate the frequency of abdominal pumping. Depression of carbon dioxide below normal levels or elevation of oxygen above normal
levels inhibits abdominal pumping, indicating homeostatic regulation of internal gas levels. Feed-forward control of abdominal pumping occurs when neural centers that control flight muscles also stimulate the central pattern generators, increasing the rate of abdominal pumping.
In a series of classic experiments, V. B. Wigglesworth demonstrated that the terminal ends of the tracheoles can contain fluid which disappears in response to hypoxia or activity. Changes in the fluid levels in the tracheoles have the potential to strongly affect the ability of the tracheoles to conduct gases, which would provide a highly significant control mechanism for the tracheal system. Further experiments by Wigglesworth suggested that the withdrawal of fluid from the tracheoles could be due to elevations in hemolymph osmotic pressure.
In summary, the tracheal respiratory system of insects is a dynamic system, capable of a tremendous range of function and fine control. This light-weight, adaptable, high-capacity respiratory system is certainly one of the major traits which underlie the ecological and evolutionary success of insects.
