Cooperative labor defines life in the social insects. Workers receive instructions from chemical signals to travel to sites where work needs to be done. Because individual workers are relatively small, energetically rewarding food sources can be profitably harvested by groups of individuals. Nest conditions may deteriorate, and more attractive, alternate nests found. Nest emigration requires communication about the quality and location of the new nest. Territorial disputes and attacks on nests abound, and cooperative defense is the most effective way to cope with threats.
The behavior that mobilizes nestmates is termed recruitment communication, commonly used by social insects to organize and accomplish work. The mechanisms that mediate recruitment communication can be understood by analyzing the behaviors of individuals and the signals they produce. Descriptions of how pheromones and other signals organize cooperation are ubiquitous. Social coordination depends on these signals. Although systems as “linguistically” elaborate as the honeybee waggle dance have evolved to communicate information about the location of food sources or nest sites, it is phe-romones that serve the primary signaling role in wingless social species such as ants and termites.
The majority of research on recruitment behavior concerns communication during foraging, defense, and nest emigration. Workers scouts for new food sources and patrol the territory of a colony. When food or a new nest is located or a competitor from an alien colony is identified, nestmates are recruited to perform work together. Scouts that have located food or a territorial intrusion return to the nest, laying a chemical trail and sometimes performing behavioral displays to alert nestmates, which then leave the nest and orient along the scout’s trail to the target area. Recruited nestmates, in turn, may continue the process by reinforcing the trail. Regulatory mechanisms involving pheromone evaporation turn off recruitment when the food has been harvested or the threat no longer exists.
Ecology influences the evolution of recruitment communication, and the adaptiveness of recruitment behavior can be studied in reference to patterns of food distribution, predation, and competition. Foraging behavior and space-use patterns are the result of community-level interactions such as interference competition, and recruitment communication is a behavioral mechanism that mediates interactions among sympatric species. Recruitment signals, in turn, are generally trail pheromones that “excite” nestmates and orient them to a locus of activity. The physical characteristics of a trail (e.g., how long it lasts as a signal) and the response it induces reflect the ecological function of the pheromone.
This article focuses on recruitment behavior in social insects, primarily in ants and termites, groups that frequently employ these communication systems and for which the greatest level of ethologi-cal, ecological, and evolutionary understanding has been achieved. The recruitment behaviors of honeybees, which are behaviorally more elaborate, are covered in a separate Topic.
PHYSIOLOGY AND BEHAVIOR OF RECRUITMENT
Exocrine Gland Sources of Recruitment Pheromones: Trail-Laying and Trail-Following Behavior
Recruitment pheromones are discharged from exocrine glands, which are anatomical structures often specialized for their synthesis and secretion. Since the first identification of the source of the trail pheromone in the fire ant by E. O. Wilson more than four decades ago, ants have served as excellent models for the study of the organization of recruitment. Thanks to the research of Bert Holldobler and Hiltrud Engel-Siegel, among others, the structure of ant exocrine glands and the function of their secretions have been described in detail for many species. In ants, the accessory glands to the sting (the Dufour’s gland and the poison gland), the pygidial gland and sternal glands, the hindgut, the rectal gland, and the tibial and tarsal glands are known to produce substances that serve to recruit nestmates (Fig. 1l . In ants in the subfamily Formicinae, the hindgut is the source of trail pheromone, which is emitted through the acidopore located at the tip of the gaster. In another diverse group of ants, the subfamily Myrmicinae, the poison gland and the Dufour’s gland secrete recruitment chemicals. Other ants rely on a variety of glands to produce trail substances. In termites, only one source of trail pheromones, the sternal gland, has been described. The structure
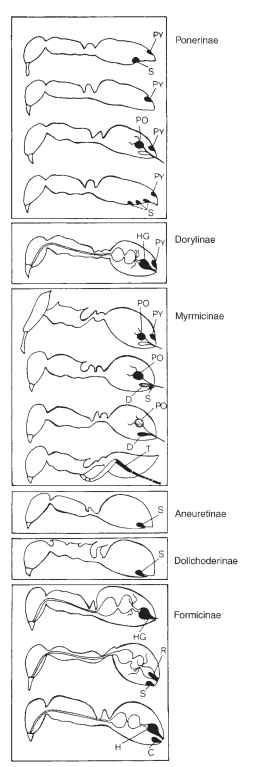
FIGURE 1 Exocrine gland sources of trail pheromones in the different subfamilies of ants: C, cloacal gland; D, Dufour’s gland; HG, hindgut; PO, poison gland; PY, pygidial gland; R, rectal gland; S, sternal gland; T, tibial gland.
of the sternal gland, which is composed of modified epidermal cells, varies in different genera. It appears that the secretions of this gland may have first played a role in defense against microbes, as the gland’s substances have antibiotic properties.
The secretions of the trail-substance-producing exocrine glands are deposited as a worker travels from a target area to the nest, or vice versa. In some ant species (e.g., myrmicine ants) the sting, serving as a conduit for the secretions of exocrine glands, is extruded and dragged over the substrate to release trail pheromones. Chemical trails may be deposited continuously or as a series of point sources between the nest and the target area, sometimes in conjunction with other secretions. The cuticle may be adapted as an applicator for the secretions of exocrine glands beneath. In termites, the sternal gland is pressed against the substrate, and sensory structures monitor contact between sternites and the substrate to regulate pheromone deposition. Some termites mark areas around the nest entrance or a food source by dotting the tip of the abdomen and laying directional trails using the sternal gland. There is significant convergence in the trail-laying behaviors of ant and termite species, both applying chemicals as point sources or streaked or continuous trails.
The spatial processing of the information encoded in an odor trail is by tropotaxis, which is a sampling of the trail pheromone by means of the paired antennae. Antennal chemoreceptors perceive variation in pheromone concentration along the trail’s semiellipsoidal active space, the area in which the concentration of the pheromone is sufficient to elicit following behavior. Theoretically, diffusion yields a gradient of odor molecules; pheromone concentration is highest at the point of application (the centerline of the trail) and symmetrically decreases on either side, defining the boundaries of the active space. The odor gradient is sampled by the antennae as the insect travels along the trail. When lower concentrations are sensed at the lateral edges of the active space, opposing movements are made so that position within the active space is maintained.
Determining the Source and Behavioral Effects of Trail Pheromones
Understanding the glandular sources and chemistry of trail substances is at the heart of the study of recruitment communication. The nature of the bioassay, the behavioral test used to determine the effectiveness of different substances as recruitment or trail phe-romones, is critically important. The bioassays used in trail pherom-one isolation and identification must distinguish among the range of behavioral responses involved in trail communication. It must be noted that trail pheromones can have both recruitment and orientation effects. A recruitment pheromone induces nestmates to leave the nest to travel to a work site. An orientation pheromone has no such stimulatory effect, but it can serve as a chemical “guide” for worker traffic. Trail substances, if they have a recruitment effect, will stimulate nestmates to leave the nest or otherwise alter their task performance in the context of a current need. In some ants, nest-mates are recruited with a motor display delivered in the nest by a recruiting worker. The trail substance in this case does not have the ability to draw ants out from the nest; rather, it is used as an orientation cue by nestmates that have contacted a recruiting ant. Some trail pheromones can alone elicit both excitation and orientation in the absence of any other behavioral display or stimulus. If an artificial trail (one prepared from a solvent extract of the appropriate exocrine gland) is drawn out from the nest entrance and ants leave the nest to follow it, a recruitment effect has been demonstrated. If the artificial trail cannot induce inactive workers to leave the nest, yet the trail is able to orient workers alerted by either a motor display or some other trail chemical that has an alerting property, an orientation effect is occurring. Careful dissection of the kinds and sequences of behaviors in the recruitment process and detailed chemical analyses have revealed that several pheromone constituents may control a number of behaviors associated with recruitment and trail following.
TRAIL CHEMISTRY AND RECRUITMENT BEHAVIOR
Social insects may mix the secretions of different exocrine glands to induce recruitment and trail-following behaviors, or the chemical output of a single gland may be composed of more than one substance, each having a distinct role in releasing behavior. Trail communication can therefore be a multisource phenomenon or a process that involves a series of chemical homologs produced in the same exocrine gland. For example, the Dufour’s gland of the fire ant is the source of a trail pheromone that induces both recruitment and orientation behaviors in workers. Dufour’s gland chemistry is varied: the constituents of this gland’s secretion regulate different behaviors, which have been called “subcategories” of trail following. These chemicals include recruitment primers, synergists, and orientation inducers. Primer and inducer substances together release recruitment and orientation behaviors. (Z,E)-a-Farnesene is the principal trail orientation component isolated from the Dufour’s gland. Another chemical fraction acts together with (Z,E)-a-farnesene to increase the effectiveness of the mixture in inducing trail communication. Homofarnesenes of presently unknown function and an orientation inducer present in the secretion also increase trail following. In the ant Myrmica, homofarnesenes in the Dufour’s gland may be added to 3-ethyl-2,5-dimethylpyrazine (EDMP), which is the poison gland trail pheromone, as part of a multicomponent trail system. Electroantennograms have been used to characterize the olfactory response to trail substance constituents.
Similar findings have been made in other ant species, and new glands and multiple sources of trail substances have been described. Pure chemicals seem to induce lower responses than gland extracts, indicating the importance of the naturally occurring chemical mixtures in trail communication. Constituents present in different ratios sometimes show syn-ergistic effects. Artificial trails prepared from extracts of the poison gland of the harvester ant, Pogonomyrmex badius, have a recruitment effect lasting approximately 20 minutes, whereas artificial trails prepared from Dufour’s gland secretions and aged for longer periods of time have elicited orientation responses. In the ant Leptogenys diminuta, the poison gland and pygidial gland produce (3R,4S)-4-methyl-3-heptanol and iso-geraniol, respectively, to regulate orientation and recruitment. Similarly, pheromone blends are known to mediate alarm communication, including alarm-recruitment systems involved in defense.
The sternal gland secretions of termites stimulate recruitment and may have highly durable orientation effects. Single chemicals such as (E)-6-neocembrene A and dodecatrienol have been isolated from whole-body extracts, but termite sternal gland secretions probably are more elaborate mixtures of pheromones that have different functions. Researchers have described “recruitment” and “basic” trails in termites; “basic” trails have only an orientation effect. The sternal gland secretion of Nasutitermes costalis, for example, can induce recruitment (drawing undisturbed soldiers and workers from the nest) and can orient searching and/or homing termites. Sternal gland material collected from trails aged for more than 20 years can orient, but not recruit, termites. Although the chemical that regulates recruitment dissipates in minutes, the orientation component of the secretion is a remarkably stable pheromone.
The persistent orientation components of a trail substance can “channel” foragers away from neighboring nests to minimize aggressive confrontations and can also serve as territorial recognition cues or as an initial guide for naive foragers. Some species of desert ants have trunk trail systems (a network of trails emanating from the nest entrance and arborizing at their distal portions) marked with Dufour’s gland secretions composed of durable blends of hydrocarbons that are specific for species, populations, and colonies. In other ant species, different glands may produce trail chemicals with different behavioral effects. The ecological significance of trail structure in termites is not well understood, although apparently foraging galleries divide foraging space to increase the efficiency of harvesting food. Recent comparative studies have examined the fine structure of sternal glands of pseudergates and workers.
Trail Pheromone Specificity
Pheromone specificity is achieved through chemical mixtures and molecular structure. Early studies in ants suggested that trail substances were highly species specific, but results of more recent work do not support such a conclusion. One striking example of this lack of specificity is that ants in as many as six different genera in the subfamily Myrmicinae use the same trail pheromone, EDMP. Dodecatrienol, a trail pheromone in the termites Reticulitermes virginicus, R. speratus, and Coptotermes formosanus, provides a nonspecific orientation cue in these species, in other species of Reticulitermes, and in a cluster of geographically and phylogeneti-cally diverse termites. (E)-6-Cembrene A, which has been isolated from whole-body extracts of the Australian Nasutitermes exitiosus, can induce orientation in African nasutitermitines, rhinotermitids, and to a lower degree in African macrotermitines (fungus-growing termites). At present, it is challenging to explain the significance of trail pheromone specificity in termites.
Metabolic end products, dietary differences, and genetics may produce variation in the chemistry of trail pheromones. In myrmi-cine ants, pyrazines and farnesenes are shared by different species and genera; these chemical constituents, present in small quantities and serving no function in one species, may have a prominent role in another. Biochemical variation may provide a substrate for evolution to act on in the selection of trail pheromones.
There have been many analyses of the level of specificity of trail substances, but the ecological significance of specificity is to a great extent unknown. Research on the behavioral ecology of trail phe-romones indicates that chemical specificity may play a role in community structure. For example, competition may have selected for differences in the trail communication systems of sympatric species. Indeed, variations in recruitment communication systems in desert ants have been correlated with resource use and competition. Desert harvester ants forage as individuals on evenly distributed seed resources and recruit nestmates to cooperate in collecting seeds from dense patches. Different foraging strategies may be adapted to the exploitation of resources with different density distributions, serving as a mechanism of resource partitioning in granivorous ant communities. Foraging systems and their pheromonal regulatory mechanisms may also enhance food defense and retrieval, thus reducing interference competition.
The behavioral mechanisms of recruitment that are the basis for enhanced competitive ability may be associated with trail phe-romone chemistry and response specificity. The harvester ants Pogonomyrmex rugosus and P. barbatus, which are very similar ecologically, have trunk trail systems that divert groups of foragers away from each other. Their trunk routes, which are composed of Dufour’s gland and are colony specific, suggest chemical differentiation resulting from competition. The specificity of the trunk routes may also give an advantage in territorial defense if fighting ability is greater when ants are on their own territory. Other ant species are known to mark trails with persistent colony-specific pheromones.
Colony specificity in the trail pheromones of termites has rarely been examined. In Trinervitermes bettonianus, workers do not discriminate their own trails from those of neighboring colonies, although their trail pheromones and the trail substances of other sympatric termites appear to be species specific. Some termites distinguish between intra-and interspecific competitors. Workers deposit rectal fluid and sternal gland secretions on trails, a mixture that may encode colony identity.
Surprisingly, the chemical trails of some ants may also encode information about the identity of the individual that laid the trail. Individually specific trail markings are used during nest emigration by workers of the ant Pachycondyla tesserinoda. Individually specific trails, used for food recruitment, have also been described in the ant Leptothorax affinis. Individuality in a trail pheromone could provide a finely tuned mechanism of directional discrimination and maintain the path fidelity of individual foragers.
If ants can decipher the chemical code of another species’ trail substance, they may be able to exploit information about the location of food sources. Interspecific trail-following is uncommon but is known in ants in parabiotic associations (i.e., different species of ants living together in a compound nest). In these species, foragers lay and follow their own chemical trails but are also able to interpret the trail pheromones of other species and to exploit their food discoveries.
Regulation of Recruitment and Foraging Activity
Trail pheromones communicate information about food quality to nestmates that have not had direct experience with a food source. Trail-laying behavior regulates pheromone concentration (i.e., the amount of pheromone deposited on a trail), which in turn controls a colony’s response. After deposition on the substrate, a trail phe-romone diffuses. The chemical properties of the pheromone both determine the spatial and temporal structure of its active space and regulate foraging activity at the colony level. The concentration of trail pheromone mediates communication between groups of individuals, those that have fed and any potential foragers within the nest. In fire ants, the continuity of the sting trail, measured by causing a scout ant to walk over a smoked glass slide (removing soot from the slide where the ant’s sting is dragged) depends upon the concentration of a sugar solution offered as a food source. The more concentrated and thus rewarding the solution, the greater the extent to which the sting is extruded and dragged continuously over the substrate, and the greater the number of workers that will lay such a trail after they have contacted the food source (Fig. 2). The distance between the food source and the nest can also affect the rate at which food is retrieved, and thus the profitability of the colony’s foraging.
The regulatory mechanism underlying foraging organization is called mass communication. Foraging is initiated when scouts locate new food, determine its quality, and deposit trail pheromone according to the food’s energetic value and the colony’s nutritional needs. This trail induces recruitment in nestmates, which repeat the cycle of food quality assessment and trail phermone deposition until the food source has been depleted or the colony satiated. The entire process is regulated by the concentration and evaporation of the trail pheromone. If the recruitment trail is not reinforced, the trail substance evaporates and foraging rate decays over time until food collection ends. Different ant and termite species show variations on the theme of mass communication that likely are associated with the foraging ecology of individual species.
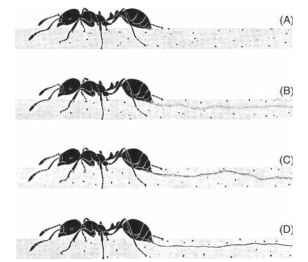
FIGURE 2 Fire ants deposit trail pheromones by discharging the contents of the Dufour’s gland through the extruded sting. The continuity of the trail is made visible when a worker walks over a treated glass slide, removing soot where her body contacts the substrate. Marks made by the tarsi and hairs on the tip of the gaster are visible. Increase in the continuity of the sting trail can be seen.
DEFENSIVE RECRUITMENT AND DIVISION OF LABOR BETWEEN CASTES
Social insects can be the most important competitors and predators of other social insects. Army ants, for example, exert significant predation pressure on social wasps and ground-dwelling ant species, favoring the evolution of adaptive systems of predator recognition and response. Some responses involve a division of labor among castes. Castes are groups of individuals specialized for a given set of tasks; they may have specific functions related to foraging or colony defense, and different castes may be recruited according to their specialization. Upon encountering a particularly important competitor or predator, ants that are patrolling the territory of a colony may preempt an attack through a caste-specific alarm-recruitment system. For example, the ant species Pheidole dentata and Solenopsis geminata are sympatric (i.e., occur together) in the southeastern United States and use similar nest sites. S. geminata, the native fire ant, as well as S. invicta, the imported fire ant, may attack colonies of P. dentata and destroy the colony (Fig. 3). Because fire ants are fierce predators of P. dentata, it is important to quickly respond to a potential threat. In P. dentata, minor workers usually care for brood, maintain the nest, and forage, whereas major workers, which have large heads and mandibles, have a significant role in colony defense. When minor workers encounter as few as one or two fire ants, they return to the nest, laying pheromone trails to recruit minor and major workers. Major workers recognize the threat as emanating from fire ants through the odor of the predator carried on the messengers’ bodies, and together with the excitatory behavior and trail substances deposited by minor workers, are recruited to the site
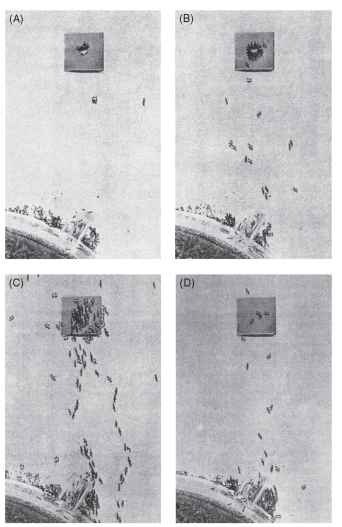
FIGURE 3 Recruitment and feeding behavior in a laboratory colony of fire ants. (A) Scout ants feeding at a drop of sucrose. (B) Ants initially recruited from the nest by trail-laying workers that had fed now feed and lay trails to the nest. (C) Recruitment increases as more ants arrive at the food and contribute pheromone to the trail. (D) The food is depleted, and new recruits arriving at the food do not lay trails on their return trip to the nest. Foraging now stops because the trail pheromone has evaporated.
at which the enemy has been located (Fig. 4). Because majors are recruited to respond defensively following contact with fire ants but not other ant species, this defensive recruitment system is said to have “enemy specification.”
Species of termites that forage above the ground face a higher degree of predation than species that forage in the confines of subterranean gallery systems, and the social architecture of these ecologically different groups of species seems to have undergone adaptive modification. For example, species whose foragers harvest food above the ground have a high proportion of soldiers that use chemical defense in combating predators. Species that nest and seek food below ground have relatively low investment in soldiers, which rely
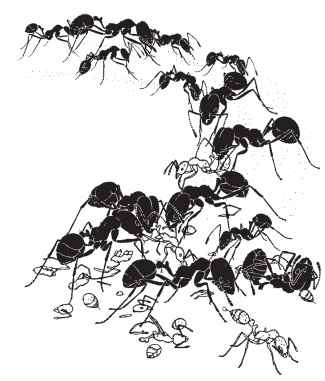
FIGURE 4 Enemy specification in the recruitment behavior of the ant P. dentata. In response to contact with fire ants (light shading), major workers are recruited to defend the colony by attacking with their well-developed mandibles.
on mechanical defense and are not involved in foraging. The recruitment communication systems that organize foraging in aboveground species appear to be adapted to reduce predation during the time period between the discovery of food and the construction of covered protective galleries, when termites are exposed and are vulnerable to predation by ants. In Nasutitermes, for example, the most diverse genus of the higher termites, there are worker and soldier castes: the ampule-headed soldiers are highly modified for chemical defense but also have an important role in organizing foraging (Fig. 5 ). Soldiers of the neotropical N. costalis scout in groups for food sources, and upon locating food communicate its location to nestmates. Groups of soldiers of N. costalis move in amoeboid fashion from the nest, ends of foraging galleries, and currently used food sources, recruiting other soldiers with sternal gland secretions as they slowly explore the environment. Parties of soldiers search as groups along trails in different areas, but all trails generally coalesce when a food source is located; subsequently additional soldiers and then workers are recruited.
There are three phases of foraging organization in N. costalis, each involving recruitment communication within and between castes. First, soldiers search for and discover new food sources and communicate information about their location to other soldiers. Next, workers are recruited in large numbers. Finally, the recruitment of workers increases further and soldiers flank both sides of the foraging trail to protect the more vulnerable workers traveling between the nest and the food. This pattern of soldier and worker recruitment shows that soldiers, which themselves do not feed directly, are
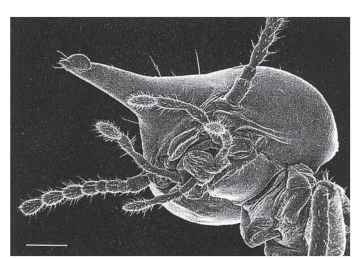
FIGURE 5 Head of a soldier of Nasutitermes. The mandibles are vestigial and the head is shaped to discharge defensive secretions. Scale bar = 0.25mm.
nevertheless scouts, which assess food quality and recruit workers that will harvest food. Soldiers first recruit other soldiers to ensure an adequate defense at the food source and then communicate with workers to begin food collection. This intercaste communication is chemical; both soldiers and workers have a sternal gland, which produces a trail pheromone that induces the recruitment of soldiers and workers depending upon the quantity of pheromone deposited. The sternal gland secretion is not caste specific, but the volume of the sternal gland varies in soldiers and workers (large workers have a significantly larger sternal gland volume than soldiers). Caste differences in pheromone perception and/or responsiveness to the sternal gland pheromone appear to regulate the prominent division of labor during foraging organization in nasute termites.
Nasute termites also have an alarm-recruitment response. When disturbed, soldiers discharge frontal gland terpenoid secretions; soldiers nearby then are recruited over short distances. Frontal gland and sternal gland secretions function in defensive recruitment and cause soldiers to remain in an area where a disturbance has been signaled. Similar foraging and defensive recruitment communication systems have been identified in other termites.
Defensive recruitment and the recognition of specific enemies occur in some termites. N. costalis responds defensively to the presence of a single soldier or worker of an alien conspecific colony by recruiting large third-instar workers, which attack the intruders with their mandibles. This response is not seen if other species of termites are encountered.
THE EVOLUTION OF RECRUITMENT COMMUNICATION
Ethological Models
Ethologists have long hypothesized that the origins of behavior can be traced from comparisons of similar actions in groups of closely related species. The history of recruitment behavior and chemical trail communication has been examined in ants with such an approach. The ancestral condition is thought to involve a behavior called tandem running.
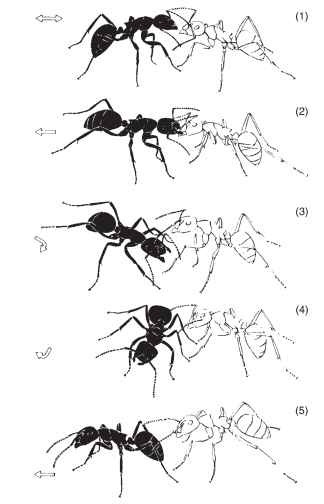
FIGURE 6 Tandem running in ants. A recruiter (black) contacts a nestmate (white) and shows a jerking behavior, pulling the nestmate by the mandibles to invite her to follow (1, 2). The recruiter then turns and offers the gaster (3, 4). When the recruited ant contacts the gaster and hind legs of the recruiter, the pair move in tandem to a new nest site (5). Arrows illustrate the direction of motion of the recruiter.
In this mode of recruitment, a single nestmate is led “in tandem” from the nest to a new nest or food source: A “leader” guides a “follower” to a target area. Tandem running involves motor displays that initiate pairing (Fig. 6) and surface pheromones and other exo-crine gland secretions to maintain the communicative tie while the ants move pairwise, outbound from the nest. This type of recruitment communication is considered to be basal because it commonly occurs in ponerine species, which are themselves ancestral in the evolutionary history of ants.
In chemical mass communication, excitatory and orienting information is contained in the structure of the trail substance, and no other signals are required to control recruitment activity. In tandem running, behavioral displays alert recruitees to the need for their assistance; chemical signals on the body of the recruiter, as well as physical contact between the members of the tandem pair, provide directional guidance. In the most derived state of recruitment communication, all information required to regulate group action is encoded by pheromones. This type of trail communication is characteristic of many species in the majority of ant subfamilies and in the termites appears to be the only method of trail communication.
The origin of trail communication in termites has received relatively little attention. The secretions of the sternal gland are known to inhibit fungal growth; thus, the ancestral function of this gland may have been the production and deposition of antibiotics to control microbial growth in the nest. Recruitment communication became more elaborate with the separation of nesting and feeding ecology and the evolution of a sterile worker caste.
Resource Distribution and the Evolution of Recruitment Communication
Some species of ants have no recruitment communication; these species offer important insights into the role of food distribution in the evolution of recruitment behavior. The relative significance of phylogeny and ecology can be separated in some of these species because the absence of recruitment communication has been noted in ants in derived subfamilies. The ant Cataglyphis bicolor, a for-micine species that nests in the ground and forages on the arid salt pans of North Africa, does not mobilize nestmates to food sources greater in size than what a single worker can transport. Recruitment does not occur naturally and cannot be induced experimentally. The diet of this ant is primarily composed of arthropods that have desiccated; the distribution of these prey is unpredictable in space and time, suggesting that the ecology of prey distribution has been the major selective force for individual retrieval without the possibility of recruitment.
Among the basal ponerine ants, the same point concerning food distribution and phylogeny can be made. Ponerine ants in the genus Pachycondyla generally show no food recruitment, employing tandem running only during nest emigration. These ants feed on randomly distributed individual prey. Yet chemical recruitment behavior occurs in species that utilize clumped food resources, such as P. laevigata, an obligate termite predator. P. obscuricornis huntresses collect prey as individuals but do not have food recruitment communication. Nest relocations involve tandem running; secretions from the pygidial gland, which is located dorsally beneath the seventh tergite on the gaster, hold the tandem pair leader and her follower together as they make their way to the new nest. In P. laevigata, an obligate termite predator, scout ants initiate the recruitment of nest-mates after only a single termite has been found. Workers of this species apply the potent secretions of their pygidial gland to the substrate by curling the gaster forward ventrally and dragging the dorsal surface (Fig. 7). One trail-laying ant can induce the formation of a foraging column of several dozen workers. Shifts in diet in the genus therefore appear to have selected for the changes in trail-laying behavior and the chemistry of the pygidial gland secretion. Another basal ant that has well-developed recruitment communication is the ambyloponine Onychomyrmex, which is a specialist on large prey and has an army ant-like life cycle.
Resource distribution is associated with the use of chemical communication during foraging in desert seed-harvesting ants. Pogonomyrmex rugosus and P. barbatus occur sympatrically and feed on seed clumps. These ants have well-developed trail communication. On the other hand, P. maricopa collects scattered seeds, primarily through individual retrieval, and has a comparatively narrow diet breadth and a relatively weak recruitment response to seed patches.
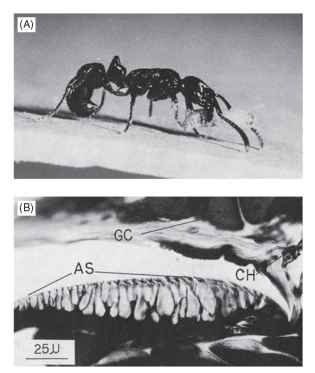
FIGURE 7 The pygidial gland and trail laying. (A) The ant P. laevigata applies the pygidial gland, located dorsally, to the substrate to lay a trail. (B) Structure of the gland: AS, cuticular applicator; GC, gland cells, CH, gland channels in the intersegmental membrane. Scale bar = 2.5mm.
RECRUITMENT, COMPETITION, AND COOPERATIVE
RETRIEVAL Recruitment communication allows the diet of a species to be expanded to include food items greater in mass than a single forager’s load size limit. This is accomplished by recruiting nestmates to help transport prey. In this way, small-bodied ants can cooperate in prey retrieval, sometimes greatly increasing diet breadth. Recruitment communication is flexible; the number of ants in the cooperative retrieval group can be adjusted to prey size and thus allow a colony to efficiently collect resources of different sizes. There may be an energetic advantage to cooperative foraging, reflected in reduced search and retrieval costs and the caloric benefit of successfully acquiring large prey. Recruitment may also improve competitive success; workers recruited to a contested resource can enhance prey defense as well as assist in transport. Short-range recruitment, mediated by acoustical and/or chemical signals, may be used to attract nearby ants to a food find. Such is the case in the foraging organization of the desert ant, Aphaenogaster cockerelli. Following short-range 0recruitment, a small group of recruits will cooperatively transport the food to the nest to minimize competition with sympatric ants.
The recruitment response of a colony is influenced by a number of ecological factors that can alter the way in which resources are partitioned between species. Recruitment varies with temperature and food item size, and each species in a community may have different thermal and prey size optima. This will cause competitive ability to vary among species and may result in some species “specializing” on prey of a certain size while
foraging within a given temperature range. In open-field north temperate ant communities, the small-bodied Monomorium minimum is more tolerant of higher temperatures and can use a mass recruitment phe-romone from the Dufour’s gland to recruit rapidly to large prey, which workers defend with a chemical repellent originating in the poison gland. M. minimum foragers also dissect prey more rapidly than sym-patric ants. Temperature preferences, recruitment, prey defense, and dissection together represent a foraging strategy that allows this ant to successfully retrieve large prey.
Although the benefit of recruitment is diet expansion, its costs lie in the time required to assemble a cooperative retrieval group. During this time, competing ant species may discover the prey and interfere with its exploitation. Indeed, the probability of interference competition from sympatric ant species can increase from 0% to 100% when prey items exceed the upper size limit for individual worker carriage. The ability to move prey is an important factor in decreasing losses to competitors.
SELF-ORGANIZATION AND RECRUITMENT BEHAVIOR
Theories of foraging strategy note the significance of time, energy, and food profitability in the evolution of feeding behavior. Social insect colonies adjust their foraging (i.e., regulate the number of workers feeding at a given food source) according to food profitability, the risk of worker loss to predation, and competition. Recruitment communication, through the physical properties of trail pheromones, allows foraging adjustments to be made adaptively. It is at the colony level that such adjustments in worker feeding behavior, or decisions, are made. The concept of self-organization has been used to explain how collective action at the level of the colony can be the result of simple rules followed by individual workers. The complex foraging activities of a colony thus result from the interactions of simple workers, whose behavior is determined by trail pheromone concentration.
Self-organization has been used to explain the foraging behavior of mass-recruiting species such as fire ants, which have large colonies composed of workers with limited behavioral repertoires. In addition, their communication systems rely almost entirely on the deposition and diffusion of trail pheromones. Computer simulations and mathematical models have been used to describe the mechanisms involved in dividing foragers between two food sources, as well as the structure of raids of army ants and the rotation of foraging columns of desert seed-harvesting ants. Self-organization theory has also attempted to explain why mass-recruiting ant species have larger colony size: the greater complexity of larger colonies and their efficiency of colony operations may be dependent upon the individual simplicity of the workers that make up these social groups. Individual simplicity may allow greater flexibility in colony-level behavior than would be possible in a social group composed of complex individuals. This reinforces the notion that social insect colonies have a decentralized rather than hierarchical system of control. It has long been known that the queen does not “hand down” orders to workers to direct colony activities.
Self-organization theory can provide useful descriptions of how efficiency is achieved through decentralization and group decision making. Studies of colony emigration in the ant Temnothorax cur-vispinosus have provided insight into the decision rules involved to organize recruitment. A relatively small number of ants are responsible for transporting nestmates to a new nestsite, either by discovering new sites independently or by being recruited through tandem running by successful scouts. There is much loss of information needed to guide naive nestmates to a new site, but even unguided ants are able to locate their new home. Tandem running is infrequently used to guide nestmates to nearby nests, but it is common when nest are more distant. Scout ants appear to use a “quorum rule” to decide when to switch from using tandem running recruitment vs. physically carrying nestmates to the new site by monitoring how many nestmates have already found the nest. This can reduce the cost of recruitment.
