Specialized Terms
latent period (LP) The time interval between when a vector acquires a pathogen and when the vector is able to inoculate the pathogen into a susceptible host.
mollicute A member of a class of bacteria that lack a cell wall.
pathogen An organism that causes disease. Certain environmental conditions may be necessary for disease to develop.
phloem Plant vascular tissues that transport sugars and other nutrients.
vector An agent that transports a microorganism from one host to another.
trypanosome A type of protozoan that has a leaf-like motile stage.
xylem Plant vascular tissues that transport water and nutrients from the roots upward.
Insects and mites can cause plant diseases or transport and inoculate viruses and microorganisms such as bacteria and fungi that cause plant disease. Insects are the most important vectors of plant viruses and the main or sole means of spread of many plant pathogens. Plant diseases spread by insect vectors can be crucially important to the profitable production of some crops. Insecticidal or biological control of vectors often do not control the diseases spread by some vectors, and so physiological and ecological relationships between vectors and the pathogens they transmit are important to understand.
The direct damage to plants caused by insect feeding (herbivory) generally is considered in a separate category from plant disease. However, some insect feeding causes plant responses that are very similar in appearance to plant diseases caused by microorganisms and may be difficult to distinguish from diseases caused by microbial pathogens such as viruses or fungi. See Phytotoxicity and Gallmaking and Insects for details on insects as direct causes of plant disease.
VECTOR TRANSMISSION OF PLANT VIRUSES
Arthropod transmission of plant viruses illustrates the complexity and variety of relationships between plant pathogens and the arthropods that transport and introduce viruses to the plants. Because plants and plant viruses cannot move by themselves, plant viruses usually have mobile vectors. Most plant virus vectors are insects, mites, or nematodes. Arthropods are not thought to be important in the spread of the smallest plant pathogens—viroids (infectious small ribonucleic acids or RNAs). Viruses consist of nucleic acid [either ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) that provides the genetic information for host cells to generate new copies of the virus (replication). In some cases the viral coat contains lipids or glycoproteins in addition to coat proteins.
Sucking insects that feed on plants’ vascular tissues (xylem and phloem) appear to be the most common vectors of plant viruses. The order Hemiptera contains the greatest number of insect vectors of plant viruses. Within the Hemiptera, aphids (Aphididae) transmit the greatest number of different plant viruses, followed by whiteflies (Aleyrodidae), leafhoppers (Cicadellidae), and plant hoppers (Fulgoroidea). Mealybugs (Pseudococcidae) and miscellaneous other hemipteran families have species that are virus vectors. Thrips (Order Thysanoptera) transmit only a few viruses, but these can be of great economic importance worldwide. Mandibulate, or chewing insects, mostly beetles (Order Coleoptera), transmit a relatively small number but varied types of plant viruses. Among the mites, the minute bud or gall mites (family Eriophyidae) are the most important virus vectors.
Vector transmission of a pathogen can be characterized with respect to vector efficiency or competence. Efficiency is usually estimated on the basis of how likely transmission is to occur during each opportunity that a vector has for transmission. Usually this is estimated by determining how many individuals of a particular species are able to transmit a pathogen to plants during a given time interval. An important aspect of transmission efficiency is that only a single insect species is known to transmit some viruses. Such viruses are said to be highly vector specific. Other viruses have less vector specificity; for example, many species of insects within a family or subfamily may be vectors. Such viruses are classified as having group specificity or low vector specificity.
Transmission efficiency often changes dramatically over time. Vectors such as the green peach aphid (Myzus persicae) transmit viruses such as potato virus Y (PVY) most efficiently within seconds after acquiring the virus from a plant. After only a few minutes or at most hours of feeding on plants, the aphids (family Aphididae) no longer transmit the virus to plants. In fact, even the aphid’s efficiency of acquiring PVY varies with time. Aphids fed only for a brief interval on the virus-infected source plant more frequently transmit virus than do aphids fed for longer intervals on the source plant. Aphid transmission of PVY is said to be non-persistent, meaning that virus transmission rapidly declines after acquisition (Fig. 1A), although airborne aphids lose transmission efficiency at much slower rates than do aphids that are feeding on plants or even probing an inert surface such as glass. Non-persistently transmitted viruses generally have low vector specificity; that is, many aphid species can transmit them. At the other extreme, the green peach aphid transmits potato leaf roll virus (PLRV) only after an interval of many hours or even days after it acquires PLRV from feeding on virus-infected plants but then continues to transmit for many days (persistent transmission). Only a few aphid species can transmit PLRV. The time required between the vector’s acquiring the virus and its successful introduction (inoculation) of the virus to a plant is called a latent period (LP).
Changes in vector transmission efficiency over time (Fig. 1) provide clues as to the nature of the relationship between the vector and the virus and help to explain how vectors transmit the virus. For example, the persistent type of transmission typified by aphid transmission of PLVR often results from the virus having to circulate within its aphid vector before it can be transmitted, explaining the delay or LP required for transmission. A plant virus, such as aphid transmission of lettuce necrotic yellows virus (LNYV), which must multiply within a vector before it can be transmitted, will typically have a median or average LP of days or even weeks. LNYV is also persistently transmitted by its aphid vectors after the LP is completed. In contrast, a non-persistently
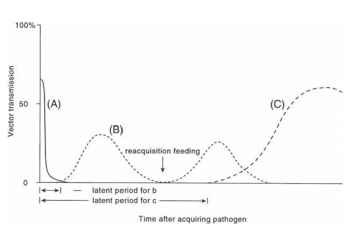
FIGURE 1 Vector transmission efficiency changes over time after acquisition. (A) Non-persistent transmission. (B) Persistent transmission. (C) Persistent transmission (over weeks), circulative or propagative transmission. The latent periods for (B) and (C) are indicated.
transmitted virus such as PVY seems to be carried in or on the needlelike mouthparts of its aphid vectors. Many such viruses produce a viral-encoded polypeptide or small protein “helper factor” to aid the attachment of the virus to the aphid vector’s mouthparts. The helper factor of one virus may also act as a helper factor for a different virus. Certain viruses may require the presence of another virus in the same plant to be transmitted to another plant. It is not known if the assisting virus or an extra-viral “helper factor” provided by the assisting virus is what aids transmission of the dependent virus. For example, rice tungro disease is caused by the rice tungro badlliform virus (RTBV), which can only be transmitted by its vector, the rice green leafhopper (Nephotettix cincticeps, family Cicadellidae), along with another virus, the associated rice tungro spherical virus (RTSV). By itself, RTBV can cause tungro disease cannot be transmitted to other plants, and RTSV by itself does not cause a disease in rice. Some viruses, such as maize chlorotic dwarf virus (MCDV), persist only for hours to days in the black-faced leafhopper vector, Graminella nigrifrons, and are classified as semi-persistently transmitted. The shedding of the vector’s exoskeleton during molting from one growth stage to another halts the transmission of non-persistently and semi-persistently transmitted viruses. Because the lining of the foregut is shed during molting, PVY and MCDV are thought to be transmitted to plants from a location within the mouthparts or foregut of the vector. Electron microscopy and the labeling of viruses with fluorescently or colloidal gold-tagged antibodies that bind to specific viral proteins have been used to identify areas where viruses accumulate or attach within the foregut. The same approach can be used for viruses that circulate within the vector’s body cavity.
The circulative viruses such as luteoviruses that are transmitted by aphids, and geminiviruses that are transmitted by leafhoppers or whiteflies do not appear to multiply within their vectors, and so their transmission is thought to entail efficient methods of viral uptake and translocation within the vector’s body cavity (hemocoel). Luteoviruses appear to be taken up by a process of endocytosis, in which virus particles are engulfed in a portion of the external cell membrane of intestinal cells, transported into the cell and expelled from the cell into the body cavity. By processes that probably involve membrane-bound transporter proteins, viruses can penetrate the membranes surrounding the salivary glands of the vector. Virus particles then enter
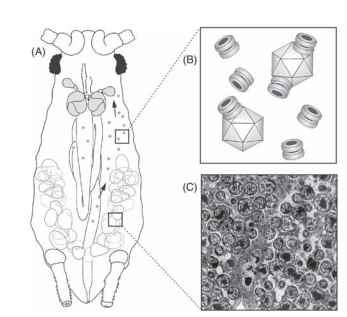
FIGURE 2 The symbionin protein of symbiotic bacteria within aphids binds luteoviruses in the hemolymph of aphids carrying the virus.
plants as a result of the vector’s salivation during feeding on plants. Luteoviurses such as PLRV can bind to the major protein (symbi-onin) associated with bacteria (called symbionts) that live in specialized tissues within aphids and provide required nutrients to their aphid hosts (Fig. 2). The attachment of virus particles to the symbi-onin molecules may aid in the efficient circulation and persistence of virus from its entry via the aphid’s digestive tract to its entering the accessory lobes of the aphid’s salivary glands. Disruption of the sym-bionts with antibiotic chemicals greatly reduces aphid transmission efficiency of the luteovirus.
Experimental manipulations can result in the “heterologous” virus made up of the genetic information (DNA) of one virus encased in the coat protein of another virus. A heterologous virus consisting of the DNA of a whitefly-transmitted virus encased in the coat of a leafhopper-transmitted virus can be transmitted by leafhoppers from plants that contain the heterologous virus to a new plant. However, once the virus begins to replicate in the plant inoculated by the leaf-hopper, it constructs the proper coat protein directed by the DNA of the whitefly-transmitted virus. Leafhoppers no longer can transmit this virus from plant to plant; only whiteflies (family Aleyrodidae) transmit it. Experiments of this sort demonstrate that the viral coat protein, not the viral genome, determined the vector specificity of the virus, probably by mediating passage of viral particles through the midgut and the salivary glands of the leafhopper, even though the viral DNA encoded whitefly-transmitted virus. Once the whitefly-transmitted virus replicated and produced its corresponding protein coat, only whiteflies could transmit it from plant to plant.
Some of the persistently transmitted plant viruses such as the reo-viruses replicate within their insect vectors. It is remarkable that the same virus can subvert the genetic and protein processing systems of both plant and animal cells for viral replication. Although vectors normally acquire viruses by feeding on virus-infected plants, some plant viruses can invade the developing eggs or embryos within a female vector insect. The rice dwarf virus (RDV) is an example of a reovi-rus that passes from virus-infected female rice green leafhoppers to leafhopper offspring by this route. Invariably these transovarially transmitted viruses such as RDV multiply within their vectors, yet most viruses that multiply in vectors are not transmitted transovari-ally. The tomato spotted wilt virus (TSWV) is unusual because it can only be acquired by immature stages of thrips. An adult thrip can transmit this virus only if it fed as a nymph on a plant with TSWV Thus the vectors of TSWV have vector specificity not only for particular vector species but also for the immature stages, at least in the acquisition phase.
ARTHROPOD VECTORS OF BACTERIAL PATHOGENS
Unlike viruses, most bacterial diseases of plants do not require insects as vectors, relying instead on rain, wind, soil, seed dispersal or other means of transport and entry to plants. However, insect vectors contribute to the spread of some bacterial pathogens of plants. Fireblight is an important bacterial disease of pome fruits such as pears and apples in which flower-visiting insects may have an important role in disseminating the causal bacterium (Erwinia amylovora) among blossoms. Insects are not essential, however, for fireblight to spread within plants once the bacteria are established, and there is little vector specificity among flower-visiting insects. Bacteria that rot potatoes (Erwinia caratovora) may be transported from infested potato tubers to uninfested tubers by flies whose maggots feed on plant roots or seeds beneath the soil. There is much greater vector specificity in corn flea beetle transmission of the bacterium (Erwinia stewartii) that causes Stewart’s wilt of corn and cucumber beetle transmission of the bacterium (Erwinia tracheiphila) that causes curcurbit wilt, an important disease of melons, squash, and cucumbers. The bacteria enter feeding wounds made by the beetle vectors, but not much is known of how the beetles introduce the bacteria into plants. Overwintering adult beetles provide an important way for these bacteria to survive the winter season without host plants.
Some bacterial pathogens are specialized parasites of plant vascular systems and require insect vectors for plant-to-plant movement and to enter and infect plants. These bacterial pathogens are specialized for vector transmission and for living in plant vascular systems. Examples are the mollicutes (bacteria that lack a rigid cell wall) that live exclusively in the nutrient-rich phloem tissues. A few bacterial pathogens with rigid cell walls, such as the bacterium that causes citrus greening disease, also specialize in living within plant phloem sap. The citrus greening bacterium is transmitted by psyllids (superfamily Psylloidea). The mollicute plant pathogens include phy-toplasmas and spiroplasmas. Most of the helical-shaped spiroplasma pathogens of plants, such as the spiroplasma that causes citrus stubborn disease (Spiroplasma citri) and the corn stunt spiroplasma (Spiroplasma kunkelii), can be cultured on artificial media. So far, none of the phytoplasma (formerly known as mycoplasma-like organism) plant pathogens have been cultured. Examples of economically important phytoplasmas are aster yellows phytoplasma in lettuce, carrot, celery, and other flower and vegetable crops and X-disease phytoplasma in stone fruits such as peach or cherry. Lethal yellowing disease of palms has been a major factor in killing coconut palms in Africa and the Caribbean region. Both phytoplasmas and spiroplas-mas are more specialized for parasitizing insects rather than plants because they can successfully colonize, and more importantly, can be transmitted by only a few species of insects. The most important vectors of mollicute plant pathogens are leafhoppers and planthop-pers, but psyllids are an important third group of Hemiptera that are vectors. The pear psylla (Cacopsylla pyricola) transmits the pear decline phytoplasma that causes the widespread pear decline disease. Typically, only one or a few species of insects within one of these families have been shown to transmit any particular mollicute. In contrast to their high degree of vector specificity, phytoplasmas and spiroplasmas can parasitize a typically wide range of plant species if the vectors can feed successfully on the plants.
Transmission appears to require that the mollicutes be taken up by vector feeding, penetrate the gut and multiply within the vector’s body cavity, enter the salivary glands, and be expelled with saliva during vector feeding into functioning phloem tissues. Thus, it not surprising that vector transmission of various phytoplasmas or spiroplasmas requires a LP ranging from 1 to over 4 weeks. The length of the LP may be very sensitive to temperature, probably because the mollicutes must multiply within the vector for transmission to occur and multiplication is temperature sensitive.
Vector-borne bacterial species that parasitize the water-conducting part of the plant’s vascular system (xylem) are less numerous but cause some important plant diseases. One such pathogen is Xylella fastidiosa, best known as the cause of Pierce’s disease of grapes, but other strains of this bacterium cause important other diseases of citrus, coffee, peach, and other crop and forest plants. Sucking insects in several families that feed primarily on xylem sap are Xylella vectors. This includes sharpshooter leafhoppers in the subfamily Cicadellinae of the leafhopper family Cicadellidae and spittlebugs (family Cercopidae). Vectors appear to transmit the bacterium from their foregut without any required LP, but continue to transmit for weeks or even months as adults. An immature vector (nymph) stops transmitting after molting its exoskeleton. Sumatra disease of cloves in Indonesia, caused by the xylem sap-inhabiting bacterium Pseudomonas syzygii, is spread by tube-building spittlebugs (family Machaerotidae), which are also xylem sap-feeders.
ARTHROPODS AND FUNGAL PLANT DISEASES
The fungi are the most varied, common, and important plant pathogens, but the great majority of fungal pathogens do not require mobile vectors such as insects or mites. Instead fungal pathogens disperse to plants mainly in wind, rain, or soil. A large variety of fungi colonize plant wounds, including those made by arthropod feeding. However, some fungi are specialized for transmission by insect vectors.
Dutch elm disease is the best-known example of a fungal disease of plants transmitted by an insect vector. The causal fungus, Ophiostoma ulmi, grows into a spore-bearing fungal mass (mycelium) under the bark and into the water-conducting woody tissues of elms. Adult bark beetles, such as the European elm bark beetle (Scolytus multistriatus) , are especially attracted to distressed elms or freshly cut elm logs. The adult beetles excavate a tunnel by feeding beneath the bark and deposit eggs along the tunnel. Beetle larvae hatch from the eggs, tunnel further under the bark, pupate, and then emerge as adults the following year. The Dutch elm disease fungus grows throughout brood chambers excavated by the beetles and produces sticky spores that attach to body and mouthparts of the adult beetles that bore out of the bark to exit the tree. The beetles transmit the fungal spores to wounds they inflict while feeding on elm twigs. The fungus gradually spreads from the point of infection into the larger branches of the tree, then to the tree’s trunk, where its action on the woody tissues eventually kills the tree. In very cold climates of North America, the native elm bark beetle (Hylurgopinus rufipes” is the main Dutch elm disease vector. Its transmission of fungal spores to elms leads to more rapid development of disease because it feeds principally on the trunk and large branches of the elm tree rather than small branches. Oak wilt disease, caused by the fungus Ceratocystis fagacearum, is spread by sap beetles (family Nitidulidae) that vector the spores from oozing cankers on diseased trees to fresh wounds on trees to which these beetles are attracted.
Some insects can create wounds which fungi can then colonize without transport by the insects. Yet even though insects in these cases are not vectors of the fungi, they can be important in determining how severe fungal infestation becomes. An example is a variety of fungi that can colonize the feeding wounds of caterpillars that feed on maize or peanuts. Some of these fungi (notably Aspergillus species) can produce powerful toxins, called aflatoxins, that sicken or even kill animals that are sensitive to the toxins. Preventing insect damage to grain in the field or storage is an important step in preventing high levels of aflatoxins.
INSECTS AS VECTORS OF TRYPANOSOMES AND NEMATODES
Plant diseases that are caused by trypanosomes are not as well understood nor as important as trypanosome diseases of animals and humans, such as malaria. Trypanosomes are protozoans of variable body shape, depending on their developmental stage and environment. Most insect-associated trypanosomes have stages that are elongated or leaf-like and are propelled by a centrally attached flagellum. Milkweed bugs (family Lygaeidae) transmit trypanosomes to milkweeds, where they harmlessly occupy the interconnected latex system. A variety of other plant-parasitic trypanosomes inhabit the phloem systems of their plant hosts, causing severe disease. Phloem necrosis disease of coffee in northern South America and heartrot of palms are spread by sucking insects in the family Pentatomidae and other related families.
Wood-boring beetles are vectors of pinewood nematodes (Bursaphelenchus xylophilus) that cause a severe disease of conifers in Asia and more recently North America. The juvenile nematodes enter the tracheae (breathing tubes) of adult long-horned beetles (family Cerambycidae), as the beetles emerge from the dead trees in which they breed. As the beetles bore into new trees, the nema-todes disperse from the beetles into the tree’s woody tissues, causing blockage of the water-conducting system.
CONTROL OF DISEASES SPREAD BY ARTHROPODS
The most obvious first step in controlling diseases caused by insect-borne pathogens might seem to be the elimination of vectors with insecticides. Although they are very effective in some situations, insecticides usually are not the best tools for control for most vector-borne pathogens of crops. The most effective approaches combine multiple methods.
Sanitation to eliminate nearby sources of the pathogen (usually diseased plants) reduces the number of infective vectors near the crop to be protected. Physical isolation to prevent disease spread may be achieved in some cases by growing susceptible crops as little as 100 m or so from infected sources, but normally much greater isolation or separation is required. Area-wide cooperation may be necessary for sanitation to control some diseases. The use of virus-free plants is probably the most widespread method of preventing virus spread. This is especially important for perennial plants such as fruit trees or plants propagated from vegetative cuttings, such as potatoes, strawberries, or sugar cane. Heat therapy or antiviral chemical treatments may be used to produce virus-free new plant growth that can be grafted or rooted to create virus-free plants for nursery propagation. As new infections of virus appear in trees, the trees can be removed; for some diseases, such as swollen shoot disease of cacao, trees within a specified radius of a newly diseased tree are also removed. Some viruses are transmitted via seeds from infected plants, and control may be based on planting virus-free seed. Lettuce mosaic virus (LMV), for example, is best controlled by using seed with less than 1 in 10,000 seedlings infected with LMV via seed. Above this threshold, aphid spread of the non-persistently transmitted LMV will be economically damaging. The production of virus-free seed may require growing the seed crop in isolated areas otherwise free of the crop and the viruses of concern. Insuring virus-free foundation plants or seed to seed producers or nurseries is an important service usually provided by government or grower cooperatives.
Keeping one period of the year completely free of the targeted crop may reduce virus spread by breaking the transmission cycle. For example, this approach has proven effective for yellows viruses of sugar beet and celery mosaic virus, both of which are aphid transmitted. The growing of these crops for several months during the year is prohibited on an area-wide basis to prevent the carry-over of virus in crop plants from one season to another. New fast-maturing varieties of rice that allowed multiple crops per year rather than a traditional single crop introduced new problems with long-established viruses because virus-infected crops co-existed next to newly planted fields. The solution was to have a least one period of the year free of all rice crops. Peak infective periods can be avoided for some virus diseases by planting after peak vector flight periods if the late planting still produces a profitable crop.
Removing diseased plants as soon as symptoms appear is an important step in preventing further spread of Dutch elm disease. The bark beetle vectors of the fungus that causes the disease are attracted to weakened trees, so removing diseased elms reduces populations of the beetles as well as reducing the percentage of beetles carrying fungal spores. Sanitation may also limit the spread of phyto-plasma diseases of trees, such as X-disease of stone fruits like cherry.
The effects of the crop environment on vector flight behavior or plant choice may be effective in slowing virus spread, even for non-persistently transmitted viruses. For example, reflective plastic sheeting used as a mulch (ground cover) repels aphids from landing on melon crops. The resulting delay in virus infection usually prevents the virus from reducing average fruit quality or yield. Sprays of 1% emulsions of paraffin oils on peppers or tomatoes reduces aphid transmission of non-persistently transmitted viruses. Plants must be sprayed frequently with oil sprays to cover new growth because the oil directly affects the inoculation and acquisition of virus by aphids.
Insecticides generally are most effective in controlling disease spread where vector-borne pathogens are persistently transmitted, are spread mostly within the crop (termed secondary spread) rather than being carried into the crop from outside sources (primary spread), or where the most important vectors reproduce on the crop. Insecticides are usually not effective against non-persistently transmitted viruses unless they quickly reduce or inhibit vector probing on treated plants. Examples of successful insecticidal control of vectors that achieved economic control of viruses are the persistently transmitted barley yellow dwarf luteovirus in grain crops, PLRV, and the leafhopper-transmitted beet curly top virus. These all are viruses that are acquired or inoculated into plants only during relatively long feeding probes by aphids. Insecticides also reduced the spread of the leafhopper-transmitted aster yellows phytoplasma if vector pressure was not too high.
Genetically based plant resistance to pathogens or tolerance of infection without loss of yield provides the basis for the most successful control programs for vector-borne plant pathogens. A drawback is that breeding resistant plant varieties that are commercially acceptable has proven to be difficult or impossible to achieve for some crops. Molecular methods of introducing novel genes for resistance to viruses directly into crop plants promises to provide resistance to virus diseases where no genetic resistance has yet been discovered.
