Specialized Terms
apomixis (-mictic) Parthenogenesis in which eggs are produced without meiosis.
automixis (-mictic) Parthenogenesis in which eggs undergo meiosis.
diploid Having two complete sets of chromosomes (like a typical adult animal).
haploid Having one complete set of chromosomes (like a typical egg or sperm cell).
haplodiploidy (arrhenotoky) A genetic system in which unfertilized, haploid eggs develop into males and fertilized, diploid eggs develop into females.
parthenogenesis (-genetic) Reproduction without fertilization (in the sense of fusion of sperm and egg nuclei).
polyploid Having more than two complete sets of chromosomes (e.g., triploid, having three sets, tetraploid, having four sets).
pseudogamy (-mous) A form of sperm-dependent parthenogenesis in which eggs require activation by entry of sperm, but only maternal chromosomes are expressed and passed on.
thelytoky (-kous) Parthenogenesis in which only female offspring are produced.
tychoparthenogenesis (-genetic) The rare or occasional production of eggs that start developing without having been fertilized.
Most insects, like most other animals and plants, reproduce sexually. Each gamete (egg or sperm) contains one complete set of chromosomes, and the fusion of a sperm and an egg results in a zygote, which then develops into a new individual. In some insects, however (as in many other groups), offspring can develop from an egg alone, without sperm, a process known as parthenogenesis (partheno-, virgin, + -genesis, origin, from gen-, to be produced).
Insects have always played a central role in our understanding of parthenogenesis; Charles Bonnet first demonstrated the occurrence of this phenomenon in the 1740s by careful experiments with isolated females of three species of aphids. During the next hundred years, further experiments with aphids and with bagworm moths, drone bees, and silkworm moths verified the reality of virgin birth, though the term “parthenogenesis” was not coined until the 1840s. Parthenogenesis has fascinated biologists since it was first described. Especially interesting are life cycles in which males and mating are completely lacking, because they call into question cherished assumptions about the importance of genetic variability in nature.
FORMS OF PARTHENOGENETIC REPRODUCTION
Thelytoky and Arrhenotoky
The technical term for parthenogenesis in which all the unfertilized eggs develop into females is ” thelytoky. ” Thelytoky represents a radical departure from ordinary sexual reproduction, and has dramatic consequences for individuals, populations, and species. It allows females to: (1) pass along their successful genotypes to all of their offspring; (2) produce only daughters, maximizing the rate of increase; and (3) eliminate the need for finding or attracting a mate. Sexual reproduction, in contrast, results in: (1) offspring with a diversity of genotypes; (2) production of males, which cannot themselves produce offspring; and (3) an inability to reproduce without males being locally present and without diverting a certain amount of time and energy to the mating process.
Facultative or obligate thelytoky occurs sporadically but is found in over 80 families of the superclass Hexapoda, and is also scattered throughout the mites (Acari: we use mites as a collective term for all acarines, including ticks). Generally, thelytoky occurs as scattered instances in hexapods, though there are several families of mites (in the suborder Oribatida) that are strictly thelytokous.
Thelytoky is found in most orders of hexapods but the orders with the highest frequency of strictly thelytokous species are Thysanoptera, Psocoptera, Hemiptera (especially in the suborder Sternorrhyncha), and Phasmatodea. The largest insect orders (Lepidoptera, Diptera, Coleoptera, Hymenoptera) have a low incidence of thelytoky overall, but very high rates of thelytoky in some families, such as weevils (Coleoptera: Curculionidae), bagworm moths (Lepidoptera: Psychidae), and chironomid midges (Diptera: Chironomidae). Thelytoky has never been reported from several species-poor orders (Protura, Diplura, Zoraptera, Grylloblattodea, Mantophasmatodea, Megaloptera, Raphidioptera, Mecoptera, Siphonaptera), or from Plecoptera, and there have been only unsubstantiated reports of thelytoky in Dermaptera, Neuroptera, and Strepsiptera.
Most of this article deals with the various types of thelytoky and their consequences. But it is important to note that there is another, very different kind of parthenogenesis found in insects and mites: arrhenotoky, in which the individuals that develop from fertilized eggs are male. Almost always these parthenogenetically produced males are haploid, and thus arrhenotoky is commonly referred to as “haplodiploidy”. In two orders (Hymenoptera and Thysanoptera), haplodiploidy is the only known sexual system, and it is widespread in prostigmatid and astigmatid mites. Haplodiploidy also occurs in some scale insects (Hemiptera: Margarodidae), white-flies (Hemiptera: Aleurodidae), some bark beetles (Curculionidae), and the bizarre beetles of the monotypic family Micromalthidae. Although arrhenotokous males arise parthenogenetically, a haplodip-loid species as a whole reproduces sexually, and the genotype of each parthenogenetically produced male is a unique recombinant product of meiosis. Thus, arrhenotoky has more in common with typical sexual systems than with other forms of parthenogenesis and therefore we do not dwell on it in this article.
One also sometimes comes across the term “deuterotoky,” meaning the parthenogenetic production of both males and females. For instance, in some species that usually reproduce by thelytoky, a very small percentage of individuals develop into males, which may or may not be able to mate. The term deuterotoky is also sometimes applied to cyclic and facultative parthenogenesis, which we discuss later in this article. For the rest of this article, and in most of the scientific literature, the word “parthenogenesis” can be assumed to refer to thelytoky.
Apomixis and Automixis
The most fundamental feature of the life cycle of sexual eukaryo-tes is the alternation between meiosis, in which the DNA content of a diploid nucleus is halved, and syngamy, in which two haploid nuclei (usually from a sperm and an egg) fuse to form a new diploid nucleus. In many insects, this ancestral cycle has been replaced by apomixis, in which there is neither meiosis nor syngamy; instead, new individuals arise from mitotically produced cells that are genetically identical (except for new mutations) to the parent that produced them. Genetically speaking, apomixis is the simplest form of parthenogenesis.
Another form of parthenogenesis is automixis, in which meiosis does occur, but eggs nonetheless develop without being fertilized by sperm. Instead of relying on sperm to restore the haploid egg to diploidy, there are a variety of different mechanisms for restoring the egg’s diploidy (Fig. 1). Some of these do so by duplicating the genome of a single cell; others by fusion of two different cells. The exact mechanism of diploidization determines whether homozygos-ity is enforced in every generation or heterozygosity is maintained for at least some time.
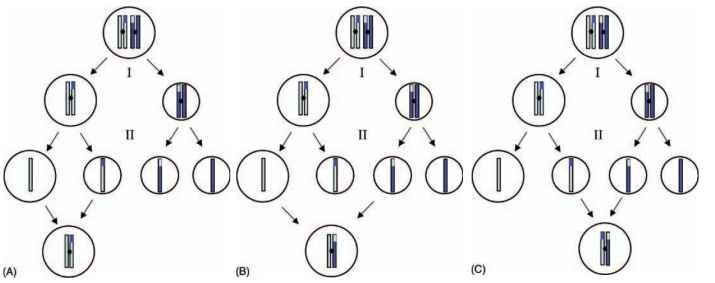
FIGURE 1 Three forms of automictic parthenogenesis and their genetic effects. For simplicity, only a single chromosome and a single crossing-over event are illustrated. The two shades of blue represent the mother’s two chromosome sets. “I” indicates the first mei-otic division, “II” the second. (A) The second polar nucleus fuses with the egg nucleus, a process called “terminal fusion”; found in some Orthoptera, Hemiptera, Thysanoptera, Diptera, and Hymenoptera. (B) A derivative of the first polar body fuses with the egg nucleus; found in Lepidoptera. (C) The two central polar nuclei fuse, “central fusion”; found in Lepidoptera, Diptera, and Hymenoptera. Note that in A, heterozygosity is lost immediately in regions adjacent to centromeres, while in B and C heterozygosity is retained in regions adjacent to centromeres. Farther from the centromere, probability of loss of heterozygosity is closer to 50%, depending upon crossing over, for all three systems. More extreme systems (retaining all or no heterozygosity) are described in the text.
At one extreme are the automictic mechanisms that are genetically equivalent to apomixis, preserving the parent’s entire genome with all of its heterozygosity. This can happen if the entire genome is duplicated before meiosis (“premeiotic doubling,” Orthoptera, and Coleoptera), or if the two products of meiosis I fuse (Collembola, Lepidoptera). At the other extreme is the instant enforcement of homozygosity across the entire genome, which occurs when the haploid egg nucleus divides and then fuses again (“gamete duplication,” in some Orthoptera, Hemiptera, Lepidoptera, Diptera, and Hymenoptera). In between these extremes are automictic mechanisms involving the fusion of two of the four haploid products of mei-osis, which result in preservation of heterozygosity at some loci but loss of heterozygosity at others. Figure 1 illustrates three examples.
All of the above forms of automixis are considered to be parthe-nogenetic in the traditional sense. However, there is another form of reproduction that involves only a single parent- self fertilization, which is the fusion of egg and sperm cells from a single individual. Only hermaphrodites, producing both eggs and sperm, can self-fertilize, and hermaphroditism is exceedingly rare in insects and mites. Indeed, only three species—all of them scale insects in the genus Icerya—are known to be hermaphroditic, and all three regularly reproduce by self-fertilization. Self-fertilization results in a loss of about 50% of the parent’s heterozygosity in every generation.
Obligate, Cyclical, and Facultative Parthenogenesis
An obligately parthenogenetic lineage is one that has no means of gene exchange with other lineages, and no means of reproduction other than parthenogenesis. But many insects have more complex life cycles that encompass both sexual reproduction and parthenogenesis. Cyclical parthenogenesis involves an alternation of one generation of sexual reproduction with one or more generations of parthenogenetic reproduction. The term facultative parthenogenesis potentially covers a number of possible life cycles, but always implies that a given individual can reproduce either sexually or asexually. In some groups of insects, sexual reproduction is the norm, but if a clutch of eggs is left unfertilized for sufficiently long, a small proportion of them will begin development. Rare overall in insects and mites, this type of facultative parthenogenesis—often called tychoparthenogenesis—is rather common in mayflies (Odonata) and in some polyneopteran orders (Orthoptera, Blattodea, Phasmatodea, and Mantodea) and also occurs in Psocoptera, Lepidoptera, and Diptera. The proportion of eggs that develop successfully into adults is low, often extremely low; hatching of unfertilized eggs of tychoparthenogenetic mayfly species varies from 4 to 8%, but for caught-in-the-wild Drosophila mercatorum, the chance of an unfertilized egg developing is only about 1/10,000. Where the cytology of tychoparthenogenesis is known, it is always automictic. Tychoparthenogenesis shows that a capacity for parthenogenesis exists within many normally sexual species, and artificial selection experiments with drosophilids and stick insects have shown that there is genetic variation underlying the capacity for parthenogenesis. The experimenters were able to increase by up to a thousand-fold the proportion of eggs that develop parthenogenetically.
The categories of cyclical and facultative parthenogenesis inter-grade when the number of parthenogenetic generations intervening between sexual ones is variable, as in certain cecidomyiid midges, all aphids, and micromalthid beetles, all of which switch from parthenogenesis to sexuality in response to environmental cues. But in cyclic parthenogenesis, there is never more than one sexual generation before the switch back to parthenogenesis occurs. Only cynipid gall wasps are regularly cyclical in the sense that there are never two successive parthenogenetic generations, although in cynipids, as in every other group with cyclic or facultative parthenogenesis, obli-gately parthenogenetic lineages have arisen multiple times.
One unusual system that deserves mention here is polyembryony. It is similar to cyclical parthenogenesis in that there is an asexual proliferation of offspring in between successive sexual generations, but polyembryony is not strictly speaking a form of parthenogenesis (which involves unfertilized eggs). Instead it is a process of “vegetative” fission of multicellular embryos. As many as 2,500 individuals can develop in this way from a single egg. Polyembryony in insects occurs regularly in parasitoids in a few families of Hymenoptera and in Strepsiptera, and has been reported to occur sporadically in grasshoppers. Polyembryonically produced siblings will be genetically identical to each other, but not to their parents.
A remarkable feature of both cyclic parthenogenesis and poly-embryony is the differentiation in some cases of a sterile “soldier” morph, analogous to the soldier caste often found in eusocial insects. In some cyclically parthenogenetic aphids, soldiers attack intruders into the plant gall that houses the aphid colony. In polyembryonic wasps, the soldiers are traditionally called “teratogenic larvae” and may attack other wasp larvae within a host caterpillar.
Mating-Dependent Systems
Thelytoky is characterized by absence of mating; indeed, the first indication that an insect species might be parthenogenetic is the observation that no males exist in collections. In pseudogamy (also called gynogenesis or sperm-dependent parthenogenesis), mating with males of the same or a different species is necessary; egg development is activated by contact with or penetration by sperm. After entry into the egg, paternal chromosomes degenerate, and only maternal chromosomes survive in the offspring. This process can be considered pseudofertilization because offspring develop clonally, and all will be female, and is thus genetically a form of parthenogenesis. Pseudogamy is difficult to observe, since mating does occur; it is usually discovered by noting that mated females regularly produce all-female broods. In insects, pseudogamy is found only in Coleoptera (two origins in Ips bark beetles and one species of pti-nid beetle), two species of Lepidoptera, and in one species each of Collembola, Orthoptera, and Homoptera. Possible cases of pseudogamy in acarines have not been confirmed.
In hybridogenesis, the paternal genome is excluded during oog-enesis such that ova have only maternally derived chromosomes. The intact maternal genome is paired in each new generation with sperm from males of a sexually reproducing host species. Such hemi-clonal reproduction was first discovered in vertebrates, and to date is known in only the genus Bacillus (order Phasmatodea). Although classed along with pseudogamy as ” sperm-dependent parthenogenesis,” it is actually a hybrid form of reproduction; it combines sexual reproduction with respect to spermatogenesis, with nonrecombinant, clonal maternal genomes.
An extraordinary system similar to hybridogenesis has recently been discovered in the ant Wasmannia auropunctata. Ants are typically haplodiploid: unfertilized eggs develop into males and fertilized eggs develop into females which may be either sterile workers or reproductives (queens). But in W. auropunctata, queens lay a certain proportion of apomictic diploid eggs which develop into the next generation of queens, as clones of their mother. Fertilized eggs develop, for the most part, into sterile workers. But in a proportion of fertilized eggs, the father’s genome replaces the mother’s completely (an unusual reversal of pseudogamy that is usually referred to as androgenesis), resulting in haploid male clones of the father. Thus, males and females both reproduce clonally, and the hybrids between them are sterile.
Finally, regular inbreeding between close relatives has genetic consequences very similar to those of self-fertilization or some forms of automictic parthenogenesis: extreme inbreeding (such as brother-sister mating) should result in homozygous lineages within a few generations. Because of this, some evolutionary biologists include extreme inbreeding under a broader definition of parthenogenesis, despite the involvement of mating. The similarity to automictic parthenogenesis is enhanced by the fact that species with regular sibling mating usually produce only one or a few males per brood, such that populations consist mostly of females. An important difference from automictic parthenogenesis is that rare outcrossing—which can have enormously important evolutionary consequences—ts likely to occur from time to time in the case of extreme inbreeders when multiple females colonize a small patch, but may be much rarer or entirely absent in automictic parthenogenesis. The taxonomic distribution of extreme inbreeding is not precisely known; it has evolved frequently in bark beetles (Scolytinae), occurs sporadically in Hymenoptera (where it has been intensively studied in parasitic wasps, but also is found in wasps, bees, and a few ants), eusocial Thysanoptera, and mites.
ORIGINS AND GENETICS OF
PARTHENOGENESIS
Relatively little is known of the details of how parthenogens arise, or of the genetic changes necessary to subvert the mictic cycle. Parthenogenesis seems often to have resulted from the genetic disturbances that accompany the intrusion of foreign chromosomes; all vertebrate parthenogens appear to have arisen from interspecific hybridization, as shown by studies of chromosomes, protein variation, and DNA sequences. A hybrid origin for thelytoky has also been shown for some of the best-studied cases in insects, Warramaba grasshoppers and Bacillus stick insects (nonhybrid thelytokes also occur, for the latter). Experimental hybridization between presumed parental species has generated parthenogenetic forms indistinguishable from naturally occuring ones, in apomictic triploid pseudogamous Muellerianella delphacid leafhoppers. Interspecific hybridization in Oncopeltus milkweed bugs also has produced pseudogamy.
However, at least three lines of evidence argue that parthenogenesis can evolve without hybridization, in at least some groups. In species with facultative parthenogenesis (such as tychoparthenogen-esis), parthenogenesis clearly arises without interspecific hybridization. Also, clonal lineages that are genetically and morphologically similar or nearly identical to known sexual species have presumably originated without interspecific mattings (e.g., thelytokous “races” of aphids and cynipid wasps that occur in otherwise cyclically parthe-nogenetic species). Finally, some species with thelytokous races are taxonomically isolated; Bromius obscurus exists as diploid bisexuals in North America but as tripoloid apomicts in Europe, and is the only species in its genus.
There is a third way in which parthenogens arise. Intracellular bacteria in the genera Wolbachia, Cardinium. and Rickettsia can induce thelytoky in various insects and mites. Remarkably, this phenomenon was predicted on theoretical grounds before it was discovered in nature. W. D. Hamilton had noted in the 1970s that maternally transmitted elements are under natural selection to bias the sex ratio as much as possible toward females. It wasn’t until the 1990s that the first case of parthenogenesis induction by maternally transmitted bacteria was demonstrated. There has been an
explosion of work on this topic since then, with many new instances of bacteria-induced parthenogenesis reported each year. In the best-understood cases, Wolbachia causes gamete duplication in unfertilized eggs, leading them to develop as diploid females rather than haploid males.
Intensive cytological and genetic investigations frequently uncover the presence of multiple mechanisms for parthenogenetic reproduction in a species or group of species. The wingless stick insect genus Bacillus provides an excellent example. Endemic to the Mediterranean region, the genus includes two sexual species, B. rossius (which also has thelytokous females) and B. grandii (strictly bisexual), and a thelytokous lineage known as B. atticus. Where two or three species occur in sympatry (as on the island of Sicily), hybridization occurs, which has resulted in the production of two hybridogens, the diploid automictic B. whitei. and the trihy-brid apomictic B. lynceorum. Each Bacillus hybrid uses a different egg maturation process; however, they share the common cytological feature of an intrameiotic extra-doubling of DNA, resulting in four-stranded chromosomes, which enables the meiotic process to produce balanced chromosome complements in gametes.
A few studies have revealed something of the genetic basis for parthenogenesis, and it is likely that it will prove as varied as are the mechanisms of parthenogenesis. In Rhopalosiphum padi (the bird cherry-oat aphid), obligate parthenogenesis appears to be determined by a single locus, and is recessive to cyclical parthenogenesis. In contrast, the predisposition for parthenogenesis in Drosophila mercatorum was induced by genes at a number of independent loci. Some lineages of R. padi reproduce largely by parthenogenesis but do produce some males (although no sexual females). There is some evidence that, in several cases, genes carried by these males have “converted” cyclically parthenogenetic lineages of R. padi into obligate parthenogens.
In many cases, what was thought to be a single parthenogenetic insect lineage has turned out to consist of a variety of genetically distinct clones, each of which was derived independently from the ancestral sexual populations. These clones may vary in ploidy and in their genetic composition, and as a result parthenogenetic populations can exhibit considerable diversity in morphology, behavior, and life history. Although much less than the variability within an outbreeding sexual population (in which each individual is genetically unique), the clonal polymorphism within such thelytokous populations implies a potential for adaptation to a wider range of ecological conditions than would be possible for an invariant population. In other parthenoge-netic populations, evidence for genetic variation is lacking, and what appears to be a single clone with a “general-purpose genotype” is able to thrive in a wide range of environments. Some insect species with very long lists of host plants appear to consist of a single parthenoge-netic lineage with remarkably little genetic diversity.
PATTERNS IN PARTHENOGENESIS: BIOGEOGRAPHY AND ECOLOGY
Many parthenogenetic hexapods and mites, whether sexual or parthenogenetic, are common and abundant. In many cases, clonal forms are more widespread than their closest sexual relatives. Frequently, parthenogens have different geographic distributions from the sexual taxa to which they are most closely related, a phenomenon known as geographic parthenogenesis. Geographic parthenogenesis in hexapods (but not acarines) often takes the form of parthenogens being closer to the poles (a latitudinal gradient) and at higher altitudes. Thus, the bisexual forms of several European weevils (e.g., Otiorhynchus scaber, Polydrosus mollis) and Solenobia bagworm moths are found only in a few alpine sites thought to have been glacial refugia, whereas thelytokous forms of the same species are widespread in central and northern Europe. Psocopteran species with both thelytokous and bisexual forms follow a similar pattern in North America.
A number of ecological patterns have been elucidated to describe the distribution of parthenogenesis in hexapods and mites. For example, parthenogenesis seems to be associated with low dispersal capabilities (with winglessness in Phasmatodea, Orthoptera, and Lepidoptera) and disturbed or ephemeral habitats (parthe-nogens often being categorized as “weedy”). Among mites, clusters of thelytokous taxa (including entire families and genera of “Endeostigmatida,” Mesostigmatida, Prostigmatida, and Oribatida) are strongly associated with soil-dwelling (particularly with stable soil horizons), and thelytokous (vs. nonthelytokous) oribatids are strongly over-represented on oceanic islands. In Collembola, too, there is a strong association between soil-dwelling and thelytoky, and the only cockroach with thelytokous races (Pycnoscelus indicus) is a burrower.
CONCLUSION
Parthenogenesis encompasses a variety of reproductive systems and is often considered synonymous with ” clonal reproduction. ” Indeed, the central feature of thelytokous parthenogenetic reproduction is that maternal genomes are normally passed on intact through a series of all-female broods. It is important to emphasize, however, that there are forms of automictic parthenogenesis in which recombination is possible, and that in pseudogamous parthenogenesis, mating is necessary even though reproduction is essentially clonal.
Parthenogenetic reproduction requires a mechanism to circumvent the normal halving of ploidy that results from gametogenesis. In insects, many mechanisms for the preservation or restoration of diploidy have evolved. Either meiosis is eliminated (apomixis) or diploidy is restored (automixis) during or after meiosis. Apomixis and some forms of automixis result in maintenance of heterozygos-ity, whereas other forms of automixis result in instant homozygosity. Far from being a reproductive curiosity, parthenogenesis has arisen in most insect groups, and many parthenogenetic species are both abundant and widespread. Many economically important pests of agriculture and horticulture are parthenogenetic. Thelytokous and pseudogamous taxa are so ecologically successful that we cannot simply view them as reproductive aberrations. The success of par-thenogenetic lineages poses something of a paradox. Either genetic variation in nature is less important than we sometimes assume, or parthenogenetic lineages are more genetically diverse than we suppose.
The study of parthenogenesis can illuminate one of the central problems in biology, that of explaining the ubiquity of sex and recombination, and the adaptive significance of the laws of genetics, by revealing when and where in nature the laws of genetics are suspended or overthrown. Insects and mites, because of their short life cycles and often large population numbers, are ideal organisms for studying the evolutionary and ecological consequences of parthenogenesis.
