Odonata (dragonflies) are paleopterous, exopterygote aquatic insects, probably more closely related to the Ephemeroptera (mayflies) than any other living insect group. Dragonfly adults are predaceous, relatively long-lived insects. Their large compound eyes, strong chewing mouthparts, long legs, and unparalleled flight capabilities are ideal adaptations for catching and consuming insect prey. Although adult dragonflies have mastered the air, the immature stage (called nymph or larva) is aquatic and usually much longer lived. Adaptation to an underwater existence has resulted in striking differences in form among nymphs, whereas adults are much more uniform in shape.
Dragonflies are quite harmless insects; they do not sting and will try to bite humans only when held captive. However, they are hosts of trematodes (flukes in the family Lecithodendriidae) in Southeast Asia, and when eaten raw, they can be a source of infection in humans (by ingestion of the metacercariae). On the whole, dragonflies are considered beneficial insects for several reasons. In both nymph and adult stages, they feed on many insects that are pests of humans and domestic animals, such as mosquitoes (Culicidae), deer flies (Tabanidae), black flies (Simuliidae), and other Diptera. They are important components of aquatic food webs and are used as indicators of ecological health of streams and lakes; in some areas, nymphs are used as fish bait or as food. They make good subjects for behavioral and ecological studies, and poets and visual artists are often inspired by their beauty and behavior. Because some species are quite large and many are beautifully colored (Fig. 1), dragonflies have become fairly popular with the public. The relatively large size and distinctive color patterns allow the identification of many species of Odonata in the field, especially through binoculars. The recent appearance of several field guides such as Dunkle’s will facilitate natural history and behavioral studies also. As dragonflies become more popular, they may join butterflies as “ambassadors” for insect conservation and appreciation.
Dragonflies are a warm-water-adapted group that probably originated in a tropical environment, as evidenced by lower present-day species diversity in cooler climates (i.e. with increasing latitude and increasing altitude). At least 75% of the world fauna is tropically distributed, although a few genera (Aeshna, Somatochlora, and Leucorrhinia) have diversified in cooler climates and their centers of distribution are located at higher latitudes and altitudes. Most high-latitude species in these genera are centered in the warmer parts of their geographic ranges, but a few species (e.g. Somatochlora) are distributed mainly north of 60°.

FIGURE 1 Examples of Zygoptera: (A) Calopteryx maculata and (B) Argia tonto. Examples of Anisoptera: (C) Cordulegaster maculata and (D) Plathemis lydia.
PALEONTOLOGY
Fossil wings of several types of predatory, dragonfly-like insects from the Carboniferous period (about 320 mya) have been found. The Eugeropteridae of the mid-Carboniferous are the most-archaic known members of the superorder Odonatoidea. They had prot-horacic winglets, but they also had pleated wings and a basal wing complex that included a cell in the shape of a parallelogram which allowed changes in camber of the wings and therefore maneuver-able flight. Apparently, the early odonatoids radiated and flourished throughout the Permian, when the large land mass Pangaea was still intact. They include the broad-winged Protodonata and Protanisoptera, and the petiolate-winged Protozygoptera. These primitive species lacked one or more of the diagnostic wing characters of all extant Odonata, including the modern basal wing complex (arculus and triangular or quadrangular conformation of veins), nodus, and pterostigma (see later) that together provide for highly maneuverable, swift flight. Some of these extinct “dragonflies” were probably the largest insects ever to have existed; for example, Meganeuropsis had a wing span of about 75 cm. The archaic odona-toids persisted until the Permo-Triassic extinction, a span of roughly 70 million years.
Although the phylogenetic relationships of the Paleozoic representatives are still poorly understood, the Odonatoidea as a whole almost unquestionably form a monophyletic group. Modern Odonata probably did not stem directly from these early odonatoids. Instead, it is probable that the ancestor of modern Odonata was similar to some of the extinct Jurassic groups (for example, the Tarsophlebiidae, with non-petiolate wings) previously placed in the “Anisozygoptera,” now known to be a nonmonophyletic grouping. Therefore, the broad wings of Anisoptera and the petiolate wings of Zygoptera are equally derived characters, which probably evolved in the Jurassic. Anisoptera first appear in the fossil record in the Jurassic (150 mya), whereas Zygoptera do not appear until the Cretaceous period (120 mya).
Another puzzling question concerning Odonata paleontology is the appearance of aquatic nymphs. The earliest fossil evidence of an aquatic nymph existence is from the Mesozoic, and it has been suggested that they were semi-aquatic before that period. However, the apparent sister group of the Odonata, the Ephemeroptera, show evidence of nymphs with gills in the early Permian at least 270 million years ago; no fossil mayfly nymphs have been regarded as terrestrial.
SYSTEMATICS
Although systematics of Odonata is relatively advanced, classification at the suborder and family level is still controversial. Until recently, three extant suborders were accepted, the Anisoptera, Zygoptera, and Anisozygoptera, with the latter group being represented by only two extant species of the Asian Epiophlebiidae. However, recent analyses indicate that “Anisozygoptera” is not a monophyletic group and that all taxa previously placed in that group are extinct. Therefore, the two living Epiophlebiidae are considered Anisoptera.
Classification at the family level also has been unstable. At present, 33 families are generally accepted (Table I), although there is some disagreement with this arrangement, especially within the Zygoptera. Molecularly derived data will undoubtedly result in further understanding of taxonomic relationships and changes in classification. Worldwide, over 5600 species of Odonata have been described. The two suborders (Anisoptera and Zygoptera) have approximately equal numbers of known species. The rate of species description has remained fairly constant throughout recent decades (on an average nearly 350 new species per decade in the 20th century), an indication that the order is far from being completely known.
Nymphs are much less well-known than adults, especially in the tropics. However, nymphs of nearly all the 427 North American species and 120 European species have been discovered, and much knowledge exists on their habitat requirements and life histories. Nymphs of approximately 30% of the more than 1200 South American species have been described.
CHARACTERIZATION AND MORPHOLOGY
Characterization of the Order
The order Odonata is characterized by having a prognathous head with chewing mouthparts, large compound eyes, three ocelli, small bristle-like antennae, a small prothorax, the meso- and metath-oracic segments fused into a large pterothorax, relatively long legs with three-segmented tarsi, two pairs of elongate wings, elongate abdomen, accessory male genitalia including the intromittent organ (not homologous with the penis of other insects) on the venter of the second abdominal segment, and one-segmented cerci. The odonate pterothorax is unusual in several features: (1) the bases of the legs are crowded forward, an arrangement conducive not to walking but to grasping; (2) the sternal sclerites make up most of the lateral and dorsal walls, with the mesepisterna meeting dorsally to form a mid-dorsal carina; and (3) the wing bases are positioned posteriorly, and the tergal sclerites are extremely reduced. All these thoracic modifications facilitate strong flight and the pursuit and handling of prey. The huge flight muscles are connected directly to the bases of the wings. Therefore, the front and hind pairs can be moved independently. The wing beat rate is relatively slow compared to neopterous insects (20-40beats s_1 vs. nearly 1000 beats s-1), but dragonflies can fly almost as fast and as agilely as any other insect. Although most Zygoptera are relatively slow fliers, they can navigate precisely amongst stems and tangles of vegetation. The nymphs are unique among Insecta in possessing an elongate, hinged labium that
TABLE I Odonata Families of the Worlda |
|||
| Family | Distribution by Continent | Genera | Species |
| Zygoptera | |||
| Amphipterygidae | Africa, Asia, North and South America | 4 | 10 |
| Calopterygidae | Africa, Asia, Europe, North and South America | 20 | 172 |
| Chlorocyphidae | Africa, Asia | 15 | 143 |
| Chorismagrionidae | Australia | 1 | 1 |
| Coenagrionidae | Worldwide | 90 | 1104 |
| Dicteriadidae | South America | 2 | 2 |
| Diphlebiidae | Asia, Australia | 2 | 9 |
| Euphaeidae | Asia | 9 | 68 |
| Hemiphlebiidae | Australia | 1 | 1 |
| Isostictidae | Australia | 12 | 45 |
| Lestidae | Worldwide | 9 | 150 |
| Lestoideidae | Australia | 1 | 4 |
| Megapodagrionidae | Africa, Asia, Australia, North and South America | 42 | 285 |
| Perilestidae | Africa, North and South America | 3 | 19 |
| Platycnemididae | Africa, Asia, Australia, Europe | 27 | 222 |
| Platystictidae | Asia, North and South America | 5 | 189 |
| Polythoridae | North and South America | 7 | 58 |
| Protoneuridae | Africa, Asia, Australia, North and South America | 24 | 240 |
| Pseudostigmatidae | North and South America | 6 | 19 |
| Synlestidae | Africa, Asia, Australia, North America | 7 | 33 |
| Thaumatoneuridae | North America | 1 | 1 |
| Anisoptera | |||
| Aeshnidae | Worldwide | 53 | 428 |
| Austropetaliidae | Australia, South America | 4 | 11 |
| Chlorogomphidae | Asia | 3 | 45 |
| Cordulegastridae | Asia, Europe, North America | 4 | 51 |
| Corduliidae | Worldwide | 39 | 242 |
| Epiophlebiidae | Asia | 1 | 2 |
| Gomphidae | Worldwide | 93 | 945 |
| Libellulidae | Worldwide | 141 | 970 |
| Macromiidae | Africa, Asia, Australia, Europe, North America | 4 | 123 |
| Neopetaliidae | South America | 1 | 1 |
| Petaluridae | Asia, Australia, North and South America | 5 | 11 |
| Synthemistidae | Australia | 7 | 43 |
| Total | 643 | 5647 | |
aNorth America includes Central America and the West Indies. Based in part on Davis, D. A. L., and Tobin, P. (1984). “The Dragonflies ofthe World,” Vol. 1, “Zygoptera, Anisozygoptera.” Societas Internationalis Odonatologica Rapid Communications (suppl.), No. 3, Utrecht; Davis, D.A.L., and Tobin, P. (1985). “The Dragonflies ofthe World,” Vol. 2, “Anisoptera.” Societas Internationalis Odonatiologica Rapid Communications (suppl.), No. 5, Utrecht; and Schorr, M., Lindeboom. M., and Paulson, D. (2000). List of Odonata of the world. is folded under the head when not in use; it may be as long as one third of the nymph body when extended to capture prey.
External Morphology
The suborders differ in two major characters. In Zygoptera (Fig. 2 ), the head is wider than the thorax and the hind wings are similar to the fore wings in basal width and the orientation of the quadrangles. In Anisoptera (Fig. 3), the head is not wider than the thorax in dorsal view and the hind wings are wider at the base than are the fore wings; also, the triangle of the fore wing lies perpendicular to the long axis of the wing, whereas it usually lies parallel to the long axis in the hind wing. Nymphs of the two suborders differ in that Zygoptera have three elongate, platelike or saclike anal gills and two comparatively short cerci (Fig. 4A and 4B), whereas Anisoptera have five pointed anal appendages (Fig. 4C and 4D) and an internal rectal gill chamber. Although the term “dragonfly” typically is used for the entire order, some authors restrict this term to the Anisoptera and use “damselfly” for the more slender Zygoptera.
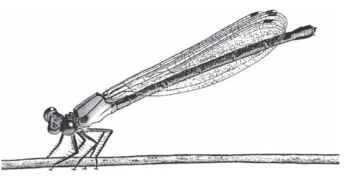
FIGURE 2 Zygoptera adult.
The wings of Odonata are richly veined to support the wing membrane. Wing venation is extremely important in systematics, especially at the family level. The beautiful colors of the body are produced by pigments under the cuticle and by diffraction of light by the cuticle.
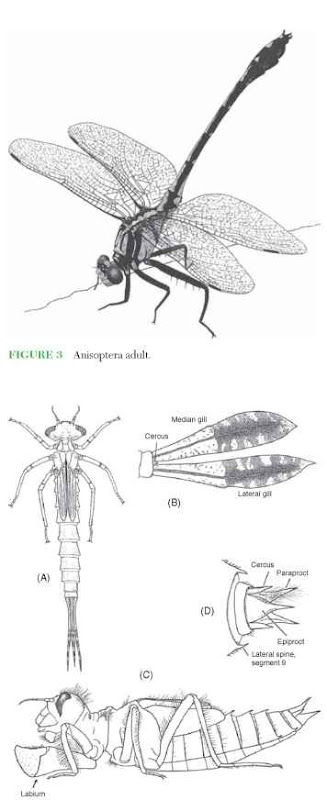
FIGURE 4 Odonata nymphs. (A) Zygoptera nymph in dorsal view, (B) anal gills of Zygoptera in lateral view, (C) Anisoptera nymph in lateral view, and (D) anal pyramid of Anisoptera in dorsal view.
Females are often less strikingly colored than males of the same species, particularly in the large families Libellulidae and Coenagrionidae. Coloring may also be produced by pruinescence, a white or bluish white exudate of the hypodermis, which forms with sexual maturation, especially in males. Nymphs are usually darkly colored, probably an adaptation to the colors of their microhabitat. In contrast to adults, body shape is highly variable in nymphs, ranging from slender cylinders to nearly flat circles, and undoubtedly reflects specific habits of foraging and escaping predation. Antennae of the nymphs are more developed than in the adults. The most-distinguishing characteristic of nymphs is the prey-capturing labium, which is highly variable in its morphology and ranges from flat to cup- or spoon-shaped and from very elongate to short and wide. The palpal lobes and prementum are armed with a highly variable number of raptorial setae, though these may be absent.
BIOLOGY
Life Cycle
Odonata are hemimetabolous. The nymphs have external wing sheaths, and although there is no pupal stage, the nymph in its final stage differs greatly in form from the adult. Several developing adult structures, such as the labium, often can be seen at this stage through the nymphal integument. Of the three life stages (egg, nymph, adult), the nymphs show the greatest diversity in functional morphology.
EGG STAGE
Eggs are laid in or above permanent or temporary water bodies. The Zygoptera and a few Anisoptera families (Aeshnidae, Petaluridae) lay their eggs in plant tissue (endophytic oviposition): most Anisoptera families lay their eggs in open water (exophytic ovi-position), although some species attach their eggs to plant tissue. Eggs are spindle shaped in endophytic species and usually round to ellipsoidal in exophytic species: they range in size from about 0.23 3 0.48 mm to 0.60 3 1.40mm. Eggs are fertilized as they pass through the female’s vagina during oviposition, and embryogenesis begins immediately after the eggs are laid. Fertilized eggs change from creamy white or light gray to a light or reddish brown or dark gray color within the first 24 h. However, eggs of some tropical species are brightly colored (e.g. blue, green, pink) throughout the egg stage. Embryos that undergo direct development hatch within 5-60 days, whereas those undergoing delayed development (diapause) hatch between 80 and 200 days after oviposition depending on temperature.
The hatching process begins several hours before actual eclosion. Peristaltic movements of the esophagus result in swallowing of amni-otic fluid, which causes water to enter the egg through the micropyles. In Zygoptera, increasing pressure within the egg causes the chorion to rupture, usually along curved lines of weakness. Continual swallowing and abdominal distention move the embryo forward, pushing the head forward into a chamber formed by the vitelline membrane. The embryo then swallows water, bursting the vitelline membrane, and the first instar slips most of the way out of the chorion, but typically the tip of its abdomen remains inside the egg. In Anisoptera, a sclero-tized frontal crest, called an “egg burster,” is used to produce a longitudinal slit in the chorion, through which the nymph exits the egg.
NYMPH STAGE
The first instar, known also as the prolarva, is extremely brief in duration. It does not feed, and the legs are seldom functional. It may last for a few seconds to a few hours, depending on whether the egg was deposited in or out of water. In most species, the prolarva molts to the second instar while the tip of the abdomen is still within the egg. In species that hatch above the water line, the prolarva drops out of the egg and reaches the water primarily by jumping. Second instars retain some yolk in their midgut to provide nutrition for a day or so, allowing them to become adept at their predatory habits. They are usually fairly pale, becoming darker in succeeding instars, usually matching their microhabitat.
The number of instars is highly variable within the order, ranging from 9 to 17. Most species have 11-13 instars, but even siblings treated identically can undergo a different number of molts. Instar classification is especially difficult with field-collected nymphs, except that the first three and last three instars usually can be determined.
Duration of each instar is also highly variable and is dependent on species, temperature, and food availability. The final instar is the longest in duration, lasting as little as five days in rapidly developing species to a year or more in others. Growth occurs immediately after each molt for about an hour, while the integument is still pliable. Growth ratios (proportional increases in linear dimension from one instar to the next) for Odonata range from 1.2 to 1.3 and are usually very close to the average for Hemimetabola (1.27). Certain body structures change with successive molts; for example, the number of antennal segments and the number of palpal and premental setae increase, dorsal protuberances on the head of early instars disappear, the compound eyes become larger, color patterns develop, and rudiments of the sexual gonapophyses appear. Wing pads usually appear during the middle instars, and grow more rapidly than any other body part.
Most species of Odonata have one or two generations per year, but many are semivoltine. A higher percentage of species are multivoltine in the tropics than in temperate latitudes; many temperate-centered Anisoptera take four to six years to complete one generation. Odonate life cycles can be classified as either regulated or unregulated. In the tropics, life cycles are regulated by alternating wet and dry seasons, whereas in temperate zones they are regulated by alternating warm and cold seasons. In regulated types of life cycles, the dry season is usually passed as prereproductive adults or as eggs, whereas the cold season is passed as nymphs or as diapausing eggs. Species that occupy ephemeral habitats undergo rapid nymphal development, but they may or may not be multivoltine, depending on other environmental conditions (combinations of photoperiod and temperature). In continuously available habitats, life cycles are unregulated.
Although a few species of Odonata have terrestrial nymphs (e.g. Megalagrion in Hawaii), the vast majority require fresh water for functions such as respiration and feeding and to prevent desiccation. Likewise, a few species occupy brackish water habitats (the libellulid Erythrodiplax berenice can tolerate truly saline conditions and occupies coastal marshes but not the open ocean).
Respiration is largely through the integument, augmented by an internal rectal chamber in Anisoptera and by external anal and rarely lateral abdominal gills in Zygoptera. Anisoptera nymphs can be readily observed breathing: as the abdomen enlarges, water is taken in through the anal opening; contraction of the abdominal muscles forces water out. The rectal epithelium of Anisoptera is developed into a specialized, richly tracheated branchial basket, into which oxygenated water is drawn by pumping action. Different families, and different genera within families, can differ greatly in tolerance of water low in dissolved oxygen. For example, many species of Libellulidae can thrive in low dissolved oxygen levels. In the family Gomphidae, nymphs of Aphylla bury deep in the soft substrate of lake and pond habitats where oxygen levels are low, whereas species in the genus Ophiogomphus lie shallow in sand/gravel substrates typically in swift, highly aerated streams. The latter group appears to have narrow environmental requirements compared with a species such as Dromogomphus spinosus, which can occupy fast or slow streams and lakes; it even colonizes newly formed ponds.
Shortly after hatching and throughout nymphal life, dragonflies must capture living prey and escape predation. In some ecosystems, they are at or near the top of the aquatic food web. Prey includes many kinds of Diptera, but probably all other kinds of aquatic insects are consumed (e.g., mayflies, hemipterans, caddisflies) as well as many other invertebrates (protozoans, oligochaetes, crustaceans, molluscs). Some species prey on small vertebrates such as larval fish and amphibians. Prey are detected usually by their movement, either tactually or visually. Motionless prey, such as snails, may be detected visually by recognition of their shape. Tactile detection is more important in earlier instars, vision becoming more keen after the first few molts. Nymphs either stalk or ambush their prey. The prehensile, protractile labium (Fig. 4C), unique to the Odonata, strikes within milliseconds to capture prey. The labium grasps the prey and brings it to the mandibles where it is chewed or engulfed whole. Because odonates are generalist predators, the potential for greatly affecting a particular prey population is low. Predators of dragonfly nymphs include fish, frogs, a few reptiles and birds, other odonates, aquatic beetles, and hemipterans. Escape mechanisms include reduction of movement, even feigning death and seeking cover. When a predator grasps a zygopteran by a gill or leg, the appendage may be autotomized and later regenerated. Anisoptera are also capable of leg autotomy, or when grasped by the head or thorax, may make stabbing movements with the abdomen, using the sharp tips of the anal appendages to deter a predator.
In general, habitats range from streams of all sizes to seeps, bogs, ponds, lakes, swamps, and marshes. Some families, such as the Calopterygidae and Cordulegastridae, are restricted to flowing waters. Within the broad habitat categories, various species occupy different microhabitats. For example, elongate, cylindrical nymphs, such as aeshnids and coenagrionids, usually cling to stems and sticks, whereas more flattened species, such as gomphids and libellulids, dwell near or in the bottom. A number of specialists occupy phyto-telmata, which includes leaf axils (such as provided by bromeliads), rotten holes in tree trunks, bamboo internodes, and depressions at the bases of large trees, mostly in tropical areas. There is great diversity in nymphal form and behavior that allows dragonflies to occupy different types of habitats. Four categories of nymphs (claspers, hid-ers, sprawlers, and burrowers) are based on how the microhabitat is occupied, although many species could be put in more than one of these categories and nymphs may move from one type of microhabi-tat to another, depending on age and season. Categorization is based mainly on leg shape and how the legs are used to situate the nymph in its resting position.
Claspers, which cling to rocks, stems, or logs, have stout curved legs and cylindrical abdomens. Hiders are less elongate and conceal themselves among dead leaves or other debris by using strong legs; they usually have many stout setae to accumulate mud particles for better concealment. Sprawlers usually are flattened dorsoventrally and lie flat at the water-substrate interface. Burrowers, which may be semicylindrical or flattened, dig into the substrate to hide; the tip of the abdomen is often elongated to protrude above the substrate for respiration. Some burrowers propel themselves through the water by forcing water out of the anus, a form of jet propulsion (e.g., Progomphus can move several inches horizontally with one pump). A very few burrowing nymphs make an actual burrow (e.g., some Petaluridae). The function of dorsal protuberances and lateral spines on the abdomen, common especially in Anisoptera, is not clear, although it is likely they serve multiple functions such as aiding in concealment and defense against attackers. Experimental evidence indicates that nymphs grow longer abdominal spines in the presence of fish predators than nymphs raised in the absence of such predators,suggesting that habitat shifts may be responsible for some of the differences observed within and between closely related taxa.
EMERGENCE
Late in the final instar, when the nymph is completing development but with a week or more before time to metamorphose to the adult stage, the wing pads begin to thicken. The final instar usually does not leave the water until the day it is to metamorphose. At this time, the pharate adult is encased in the exuviae of the final instar. The main requirement for the dragonfly at this time is to find proper support structure, as it must cling tightly in order for the metamorphosis to proceed successfully. The emergence support can include any physical object ranging from the horizontal, terrestrial substrate adjacent to the water body to upright (vertical or inclined) objects such as rocks, plants, or synthetic structures. Shortly after leaving the water and securing a support grip, the dragonfly splits the integument along the middorsal line of the thorax and begins to push the adult thorax through the narrow opening. A slit in the integument of the head then appears, and the head and thorax push out. Shortly afterward, the legs, wings and anterior portion of the abdomen appear. The dragonfly then remains motionless with legs folded for 10-20 min, hanging downward if oriented vertically, or protruding upright if oriented horizontally. When this apparent rest period is over, the legs are extended to grasp the exuviae and the rest of the abdomen is quickly withdrawn. Usually the cloudy wings then expand, followed by lengthening of the abdomen, each process taking about 15 min. As the wings become clear, drops of water are emitted from the anus and the abdomen becomes more slender, slowly taking final adult shape. The wings are suddenly spread out horizontally, and begin to vibrate. The full emergence process lasts from about 30 min to 2 h, ending with the maiden flight.
Some species emerge at night, apparently to escape predation, although many emerge at dawn or in full daylight. Some species have relatively synchronized emergence, all individuals within a population emerging within a day to about 2 weeks of each other. Other species are unsynchronized, emerging throughout the warmer seasons. Periodic exuviae collections can reveal such trends in emergence curves and population size. In nearly all species studied, the ratio of males to females is close to 1:1, although usually a slightly higher percentage of males occurs in Zygoptera, contrasted by a slightly higher percentage of females in Anisoptera.
Adult Behavior
The two main phases of adult life are the prereproductive (or maturation) period and the reproductive period. The prereproduc-tive period lasts from the completion of emergence to the onset of sexual maturity. A brief postreproductive period, after reproductive capacity has passed, is sometimes observable.
PREREPRODUCTIVE PERIOD
Upon reaching safety following the maiden flight, dragonflies remain in a teneral condition for about a day, during which time colors begin to develop and the integument begins to harden. The prereproductive phase then lasts from 2 days to several months, depending on species and environmental conditions; females usually take slightly longer to mature than males. During the prereproductive period, the gonads mature, the thoracic musculature becomes fully developed for agile flight (necessary for attaining a mate), weight increases, and mature colors are attained (for sex and maturity recognition in some species).
REPRODUCTIVE PERIOD
Males and females of nearly all species mate with more than one individual, although monogamy has been reported in the coenagrionid genus Ischnura .
Male and Female Encounters Vision is the main sense used by Odonata for finding mates. The sexes usually meet at or near the aquatic oviposition site. Males that occupy a fixed territory can be categorized either as “fliers,” patrolling continuously along the proper habitat, or as “perchers,” making short defensive flights from a convenient perch. Territories are maintained by flying at or even clashing with intruders, and pursuit often results in both individuals leaving the habitat temporarily. Resident males usually return quickly to their territory. Mating usually occurs when a female arrives at the water, although males will attempt to acquire females several hundred meters from an oviposition site.
Recognition of Conspecifics Males recognize females mainly by color, color pattern, body shape, and flight style. Males of most species directly pursue and attempt to grasp any female that comes within sight, and if successful in achieving tandem, will attempt immediately to initiate copulation. In such species, males sometimes take heterospecific females into tandem.
Courtship has been described in a few families (Calopterygidae, Chlorocyphidae, Euphaeidae, Hemiphlebiidae, Platycnemididae, Libellulidae). In some species within these families, some males (but not all) present color and/or posture displays to induce a female to copulate. For example, in Perithemis tenera (a small North American libellulid with sexual wing-color dimorphism), males establish territories around oviposition sites that consist of some sort of vegetation protruding from the water surface. On detecting a female near his site, a male will fly toward her and follow, moving from side to side. He then turns and flies to the oviposition site. The female may or may not follow him, depending on the suitability of the site. If acceptable, she will follow, whereupon the male hovers over the site, fluttering his wings. The female slows her wing beat frequency and may even perch on the site. At this signal, the male initiates tandem linkage and copulation follows. When females are unreceptive to a male’s approach, they perform distinctive refusal behaviors. Female Anisoptera usually fly very rapidly or erratically to escape, although some simply hide from males. Female Zygoptera usually show refusal by remaining perched and spreading their wings, sometimes also raising the abdomen or curving down the posterior portion of the abdomen. Males respond to such displays often by leaving, although some still attempt to achieve tandem.
Tandem Linkage and Sperm Transfer The postures adopted by Odonata in male-female tandem linkage and in copulation are unique. Tandem linkage, a necessary precursor of copulation, is initiated by the male. Males land on and seize females usually in flight (Fig. 5A), although they may grasp perched females also. The male, after landing on the female’s pterothorax, brings his abdomen up and forward so that he can grasp the female’s head or thorax with his anal appendages; he then lets go with his legs and straightens his body, thus having achieved tandem linkage (Fig. 5B). In most Anisoptera, the male appendages fit over the dorsum and rear of the female head, whereas in Zygoptera they fit over the dorsum of the female protho-rax. In the anisopteran family Aeshnidae, the male cerci fit tightly on the rear of the female head but also touch the anterior part of the pro-thorax; in some Zygoptera, the male cerci touch the anterior portion of the pterothorax. Shortly after achieving tandem, the male transfers sperm from the gonopore on abdominal segment 9 to the penis on segment 2 (Fig. 5C); this act is called intramale sperm translocation. Males sometimes translocate sperm before acquiring a female. The tandem pair either copulates in flight (most Anisoptera) or flies to a perch to copulate (Zygoptera and several Anisoptera families). The female swings her abdomen forward from underneath so that her

FIGURE 5 Mating sequence of Odonata (male with dark markings, female pale): (A) male grasping female, (B) tandem linkage, (C) intramale sperm translocation, and (D) copulatory wheel.
genital aperture engages the venter of segment 2 of the male; the pair is now in the copulation wheel (Fig. 5D). Sperm are then transferred from the male’s intromittent organ to the female’s sperm-storage organ. Copulation is usually extremely brief in flight (3-20 s) but can last from a few minutes to over an hour in perched pairs.
The male intromittent organ of Odonata is designed not only to inject sperm into the female, but also to remove or displace sperm of previous males. Jonathan Waage’s discovery of this dual function led to evolutionary understanding of the whole suite of reproductive phenomena, from sexual selection to sperm competition and mate-guarding. Sperm displacement may be achieved by removal, repositioning, or dilution. The structure and shape of the penis are vital to the mechanism employed: penes with backwardly directed barbs or hooks remove sperm from the bursa copulatrix, whereas those that are rounded pack sperm. Because most eggs are fertilized with sperm from the most-recent insemination as they pass the female’s fertilization pore during oviposition, the last male to copulate with a female is most likely to leave progeny. This phenomenon, termed sperm precedence, explains why males guard females after mating with them. By such postcopulatory association, a male protects his genetic investment by preventing other males from overtaking the female, and by inducing the female to deposit (and therefore fertilize with his sperm) most of the eggs she is carrying.
Oviposition
When copulation is terminated, pairs may break tandem linkage or remain together. Oviposition usually takes place shortly after copulation regardless of whether the tandem linkage is maintained, although females do lay eggs at times when males are not at oviposition sites. Ovipositing late in the day is a fairly common way by which females avoid interference from males. Males of many species guard females with which they mate, either by maintaining tandem contact with or by remaining near them, or both. Attempts to guard females are not always successful, as intruding males sometimes grasp and copulate with guarded females. Typically, guarded females oviposit more rapidly than those unguarded; their fitness thereby is enhanced.Contact Guarding Males of many Zygoptera remain in tandem with their mated female while she oviposits, even when she submerges.
Males of many Coenagrionidae project vertically in the air with legs and wings folded, their only support provided by the grip of their anal appendages on the female thorax. In many Libellulidae, male and female fly in tandem low over the oviposition site, the male lowering his abdomen to cause the end of the female’s abdomen to dip into the water and release eggs.
Noncontact
Guarding When males do not maintain females in tandem, they guard females by flying or perching nearby and warding off any intruding males. Males display toward and chase intruding males; male-to-male body and wing clashes may ensue. Some males guard multiple females. When male density is high, intensity of guarding ovipositing females probably increases in most species, but it has been reported to decrease in at least one species. In many Zygoptera, the male takes the female in tandem to the oviposi-tion site, then releases her, and either does or does not guard her. In some Trameinae, a subfamily of Libellulidae, the male releases the female as they fly in tandem over the oviposition site; she drops down to the water surface and releases a few eggs, then she flies back upward and the male takes her back into tandem. These latter two cases illustrate combinations of contact and noncontact guarding.
FORAGING
Odonata feed on living prey throughout their adult life. When foraging, dragonflies can be categorized as “perchers” or “fliers.” Perchers spend much of their time stationary, making short flights from perches to capture prey and then perching to consume it. In contrast, fliers are on the wing for a large part of their feeding activity, capturing their prey in the air and swallowing small prey while in flight; they do however perch to consume larger prey. The “flier” mode requires much more energy, but fliers are more opportunistic feeders, able to forage later in the day. Perchers typically capture most of their prey during midday. This dichotomy of foraging styles results because fliers generate more body heat than perchers and can therefore remain active at lower air temperatures.
The major stimulus for detecting prey is movement. Odonata have very large eyes with many ommatidia, a specialization for detection of movement. However, a few species take stationary prey, apparently recognizing the prey by its shape. Prey such as small flying insects may be captured directly with the mouthparts, but the legs are also used for subduing certain types of prey. Odonata are typically gen-eralists with few exceptions. Diptera, especially mosquitoes and midges, are a major component of the adult diet. One analysis found that Chironomidae constituted a significantly higher proportion of the gut contents than did Culicidae, probably reflecting differences in the flying and perching habits of midges and mosquitoes. Some species take mainly large prey, such as Lepidoptera and Odonata. For example, the large North American gomphid Hagenius brevistylus often has been observed feeding on other Anisoptera and has been dubbed the “Dragonhunter.” Members of the Neotropical family Pseudostigmatidae are specialist feeders. They glean small spiders in the rain forest by vertically searching trees, hovering near webs found on leaf tips, then flying directly up to the webs and snatching the spiders from their perches.
THERMOREGULATION
Although insects are basically ecto-thermic, large species are able to generate body heat or adopt body positions to absorb sunlight and are able to maintain this heat gain via certain behavioral mechanisms. Odonata possess both ecto- and endothermic thermoregulatory capabilities. The two basic behavioral styles, fliers and perchers, use different strategies to prolong activity under less than optimal ambient temperatures. Under cool conditions, fliers warm the thorax by wing-whirring (endothermy),whereas perchers expose as much of the surface area as possible to solar radiation. In some species of perchers, hairs on the thorax serve as insulators, or the wings may be deflected downward to insulate the thorax. Such species are among the first to appear in the spring at higher latitudes. Under very warm or hot conditions, perchers remain stationary longer and set their body posture to absorb less solar radiation. A common posture is the obelisk position, in which the abdomen is raised to expose the minimum surface area to the sun and the wings are lowered to reflect sunlight away from the thorax. Fliers generally become inactive during midday and hang up in the shade. However, some species of Libellulidae glide, and some species of Aeshnidae are able to continue flying by shunting warm blood from the thorax to the abdomen, where excess heat is dissipated. By prolonging activity at the breeding site, dragonflies increase their chances of obtaining mates, feeding, and escaping predation.
Dispersal
Most flight involves small-scale, intrahabitat movements for immediate needs (feeding, finding mates, and escaping predators) that directly affect individual survival and reproductive success. Such flights usually result in dispersal distances up to a few hundred meters. Large-scale flight resulting in interhabitat displacement is regarded as migratory flight. Corbet defined migration as “spatial displacement that typically entails part or all of a population leaving the habitat where emergence took place and moving to a new habitat in which reproduction ensues.” These dispersal movements also have consequences for survival and reproductive success.
In examples of migration so far elucidated for tropical species, migration is a means of overcoming drought in the area where the species developed. For example, in ephemeral lentic habitats in Africa, the aeshnid Hemianax ephipypiger develops rapidly (within 60-90 days) and upon emergence flies with rain-developing systems several hundred kilometers to areas which will receive the rainfall, as far north as Europe. There they feed, mate, and lay eggs in newly filled water bodies. Temperate species migrate to circumvent cold temperatures. For example, the wide-ranging aeshnid Anax Junius emerges early in the year in southern North America, and arrives in the northern United States and southern Canada during warm periods as early as March and April. These immigrants mate and lay eggs in shallow lentic habitats; their progeny complete development in late summer or fall. The second generation then flies south; large numbers of “green darners” have been observed flying overhead as late as mid-November. Migratory flights may be made up of several species, usually from the families Aeshnidae and Libellulidae. Very few Zygoptera are known to migrate, and all recorded so far are in the subfamily Ischnurinae (Coenagrionidae).
ODONATA SURVIVAL IN A
CHANGING WORLD
Habitat creation, loss, and alteration are the major causes of odo-nate population changes. Some odonate species have increased their geographic ranges and population numbers in response to man-made changes in habitat. For example, in the United States many pond-dwelling Libellulidae that were historically centered in the eastern part of the country have moved far west of the Mississippi River with the advent of irrigation. Furthermore, exotic species can immigrate when gravid females ride tropical storms or can be introduced as eggs and nymphs with the aquarium trade, their ranges thereby increasing dramatically. Alternately, many riverine and wetland species have undoubtedly declined because of habitat degradation and drainage changes. For example, some riverine Gomphidae are extremely rare, but could be protected by conserving the remaining habitat (e.g., in the eastern United States, Ophiogomphus edmundo is known from three localities, and Gomphus sandrius is known from seven localities). Only one species in the United States has federal protection status as a threatened and endangered species, Hine’s emerald (Somatochlora hineana). Many species in this genus are locally distributed, inhabiting lakes and bogs in different stages of succession, and their populations depend greatly on the availability of the proper microhabitat.
Awareness of threats to dragonfly diversity and populations of sensitive species exists in most countries, especially in Europe and Japan, where protection efforts are designed to heal or prevent damaged ecosystems. In tropical areas, however, where diversity is highest and incompletely known, habitat destruction continues at alarming rates. There has been some effort toward habitat conservation for all organisms, as national parks and preserves have been established in many tropical countries. For example, in Thailand, almost all remaining forest areas are protected by parks, wildlife sanctuaries, and a ban on logging; this effort amounts to nearly 15% of the total land area. Such efforts will help protect dragonflies. In other countries, the situation is not so promising, and even preserves afford no insurance against habitat alteration. Odonata have existed for many millions of years, undoubtedly surviving small and massive extinction episodes; however, the comparative magnitude of present-day environmental change is unknown.
