Neuropeptides (Nps) are bioactive peptides of neuronal origin, found throughout the animal kingdom, forming a most structurally and functionally diverse group of compounds. Nps have been well conserved during the course of evolution, indicating their major role as regulators of physiological processes. Nps are extracellular chemical messengers that act as circulating neurohormones, as local co-transmitters or as neuromodulators, and in the hierarchy of entities that regulate endogenous biochemical control functions, the Np messengers rank the highest, regulating almost every aspect of life. The original definition of Nps covered small molecules (less than 50 amino acids) of a peptidic nature, synthesized in specialized nerve cells, known as neurosecretory cells (NSCs), most of which are located in the cerebral ganglia (brain), and are released from their axon terminals, either into the intracellular space of an adjacent cell (nerve, endocrine, or non-endocrine) or into the circulatory system. Those released into intracellular spaces affect proximal effector sites; those that enter the general circulation reach peripheral organs, where their activity can be manifested either directly by activation of a distal target organ or indirectly via signals to non-neuronal internal secretory glands. In recent years, it became apparent that Nps are also synthesized in neuronal cells that are not NSCs as well as in non-neuronal cells, and the Np concept has been widened to include peptides that serve to integrate the brain and other tissues for the maintenance of normal physiology, homeostasis, and behavioral patterns.
In insects, Nps regulate a long list of physiological and behavioral processes during development, reproduction, and senescence, and they maintain growth, homeostasis, osmoregulation, water balance, metabolism, and visceral activities. In the past two decades, many insect Nps have been identified, the basic principles of their action (e.g., biosynthesis, processing, release, transport, activation of the target cell, and degradation) have been revealed, and their roles in the physiology of organisms have been determined by means of genome sequencing, peptidomics, gene microarrays, receptor characterization, and targeted gene interference, all combined with physiological, electrophysiological, and behavioral analysis. This article describes the current knowledge of the structure of the insect neuroendocrine system, including the distribution of insect Nps and their receptors, and lists the various Np families and their diverse functions.
NP RESEARCH IN A HISTORICAL
PERSPECTIVE
The concept of neuroendocrine control dates back to the beginning of the 20th century when Stephan Kopec first suggested that metamorphosis in insects is regulated by brain factors that are released into the hemolymph. Further progress in the field came from the studies of Berta and Ernst Scharrer who introduced the basic concepts of neurosecretion and NSCs, and described the similarities between the retrocerebral complex in insects and the hypothalamic-hypophysial system in vertebrates. Although neuro-secretion was first observed in insects, invertebrate Np research has lagged behind vertebrate studies, mainly because of low availability of biological material and the lack of sensitive techniques for isolation, sequencing, and lack of sensitive techniques for isolation and sequencing of peptides. The development of chemical, biochemical, and genetic engineering technologies, as well as the growing awareness of the major role Nps play in the physiology of organisms, stimulated active interest in insect Np studies, and indeed, in 1975 Starratt and Brown released their pioneering publication announcing the initial determination of a primary structure of the insect Np, proctolin. Since then, over 150 insect Nps have been identified, most of which have been isolated from the fruit fly, Drosophila melanogaster, cockroaches (e.g., Leucophaea maderae, Periplaneta americana), locusts (e.g., Locusta migratoria and Schistocerca gregaria), moths (e.g., Manduca sexta, Bombyx mori, and various Heliothine species), and mosquitoes (e.g., Anopheles gambiae and Aedes aegypti).
A variety of methods have been employed to identify the structures and to localize and study the mode of action of Nps. The first insect Nps were identified (prior to the genomic/proteomic era) by “classical” methods, namely, isolation and Edman degradation sequencing, and their putative functions were tested in different i n vivo and i n vitro bioassays. This approach was applied mainly to functionally identified Nps. Later on, Np primary sequences were predicted, based on their cloned genes, and localization studies were carried out using immu-nohistochemical and i n situ hybridization techniques. In parallel, Np receptors, obtained from fractionated cell membranes have been studied by means of specific binding assays, but only a few (less than five) had been characterized before the late 1990s.
In the past few years, research on insect Nps has seen several major advances that have accelerated our familiarization with their structures and functions. One of the most important of these advances was the sequencing of several insect genomes (D. mela-nogaster, Apis mellifera, B. mori, A. gambiae, and A. aegypti), which provided information about Np precursor genes: information that formed the basis for predicting and identifying many more Nps. Currently, 30-40 genes encoding Np precursors have been predicted in each species. The genomic information also extended our knowledge on Np receptor genes (G-protein-coupled receptors, GPCRs), Np prohormone processing enzymes, and several other genes that encode proteins which play a role in the regulation of cell-specific Np expression, thus providing us with great resources for gaining a better and deeper insight into Np functions in insects. More recently, mass spectrometry (MS) followed by sequence determination, was employed for Np identification and localization. This method enabled analysis of Nps (in the fentomole level or less) in the hemolymph, in tissue extracts and single cells by matrix-associated laser desorption/ionization (MALDI) MS and examination of neuronal homogenates by electrospray ionization (ESI) techniques. A variety of MS-based approaches (direct tissue MS or MS in combination with laser capture microdissection) have accelerated the rapid and accurate identification of predicted Nps in small amounts of tissues, and even in single neurons, and have led to both identification and localization of hundreds of new Nps in a single step.
Currently, Np studies focus on: re-screening of genome databases with novel search algorithms (database mining) to enable putative GPCRs or preprohormone sequences to be identified and then cloned from any nascent, unidentified genome; MS probing of tissue extracts for confirmation of predicted sequences; MS analysis of single neurons, in conjunction with immunocytochemistry (ICC) for localization and determination of the anatomical organization of Nps inside and outside the insect’s central nervous system (CNS); molecular manipulations at the Np gene level, for determination of the functional role of a given Np; and development of cell-based and/or cell-free recombinant GPCR-binding assays to determine the mode of action of a given Np at the cellular level, to discover orphan receptor Nps ligands, and to screen libraries (chemical, phage display, and natural compounds) for discovery of Np agonists and antagonists. Special attention is paid to the role insect Nps play as potential targets for pest management and as a basis for development of insect-control agents by means of rational/structural design approaches.
ANATOMY OF THE NEUROENDOCRINE
SYSTEM IN INSECTS
The cellular distribution of Nps and their anatomical neurochem-ical organization in insects have been studied mainly by morphological methods such as ICC and retrograde nerve filling and, in several cases, by i n situ hybridization. Recently, with the introduction of a variety of MS techniques, mass spectrometric analyses have been employed in conjunction with the above methods to identify Nps and their possible co-localization in single identified neurons of neu-rohemal organs. The studies revealed great diversity among insects in the distribution patterns of the various Nps, with diverse degrees of complexity in the various peptidergic circuits. Insect Nps are found in NSCs, interneurons, and endocrine cells. The vast majority of known insect Nps are found in NSCs that terminate in the brain-subesophageal ganglion (SOG)-corpora cardiaca-corpora allata complex (Fig. 1). This complex comprises six clusters of NSCs: a pair of medial NSCs that originate in the pars intercerebralis (PI), a pair of
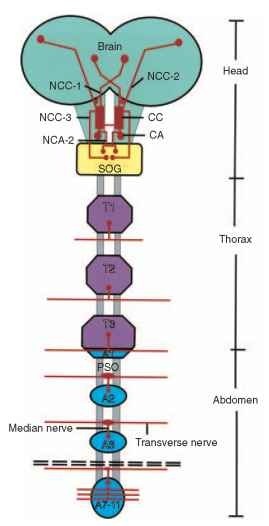
FIGURE 1 A schematic representation of the major neurohemal release sites of the CNS of insects. CC, corpora cardiaca; CA, corpora allata; SOG, subesophageal ganglion; NCC, nervus corporis cardiaci; NCA, nervus corporis allati; PSO, perisympathetic organs; T1-T3, thoracic ganglia; A1-A11, abdominal ganglia.
lateral NSCs that originate in the protocerebral region of the brain, and a pair of NSC that originate in the SOG. These six clusters form axon bundles termed nervi corporis cardiaci (NCC): NCC1, NCC2, and NCC3, respectively. Each of the nerve bundles terminates in a pair of retrocerebral neurohemal glands, termed corpora cardiaca (CC). The nerve terminals form the storage lobe of the CC through which Nps are released into the circulatory system. Additional neu-roendocrine (intrinsic) cells are present in another lobe of the CC glands (the glandular lobe), which is the site of synthesis and release of other Nps. Two additional clusters of NSCs, which originate in the SOG, extend axons that form the nervi corporis allati 1 (NCA1), which terminate in another pair of endocrine glands (of non-nervous tissue origin), termed corpora allata (CA), and form another neuro-hemal region. Another pair of dorsolateral NSCs that originate in the PI region of the brain extend axons that terminate in the CA. The CC and CA are adjacent glands, partially fused with the ventral wall of the aorta, which enables release of the Nps that are synthesized in the neuroglandular cells, as well as those from the brain/SOG nerve terminals, into the circulatory system.
Studies carried out in a variety of insects (the fruit fly D. mela-nogaster, the desert locust S. gregaria, the cockroach P. americana, and the moth M. sexta) revealed that many Nps are also found in a large variety of interneuronal cell types scattered in all areas of the brain, and that most Nps are expressed in at least a few interseg-mental neurons throughout the length of the ventral nerve cord. Np-containing cells have also been reported in visceral organs such as the gut, oviduct, accessory glands, and even hemocytes.
Another much smaller neuroendocrine structure comprises the segmentally arranged perisympathetic organs (PSOs); it serves as the storage and release site of Nps produced by neurons in the thoracic and abdominal ganglia of the ventral nerve cord. Most of the Nps that have been detected in the PSO are not homologous with those found in the retrocerebral complex. Another neuroendocrine structure is the epitracheal system; it consists of segmentally arranged nerve cells located at the trachea, near the spiracles that form the epitracheal glands (EGs). The system produces two blood-borne Nps that trigger pre-ecdysis and ecdysis behavior: pre-ecdysis-triggering hormone (PETH) and ecdysis-triggering hormone (ETH). Other NSCs are distributed throughout the insect body: throughout the CNS, in the sympathetic nervous system and the peripheral nervous system, on the aorta, and at the ampullae of the antennal heart. Most of these release sites are situated in well-circulated regions of the body. A detailed review on insect Np localization was presented by Nassel and Homberg.
NP FAMILIES
Accumulation of information on the primary structures of insect Nps led to synthesis of many peptides, which together with the availability of a large variety of bioassays enabled studies of their structure-activity relationship (SAR) and their arrangement into either structurally homologous peptide families or by their functions. Categorization of Nps into ” functional families ” was usually based on their main action or the one for which a given Np was best known. The major groups of Nps involved in development, reproduction, homeostasis, metabolism, myotropic activity, and coloration are listed below. Some peptides are involved in several functions and are, thus, listed in more than one category.
Developmental Nps
The main Nps in this category are the allatotropins/allatostatins, which stimulate/inhibit synthesis of juvenile hormones by the CA; PETH, ETH, crustacean cardioactive peptide (CCAP), and eclosion hormone (EH), which are involved in controlling pre-ecdysis and ecdysis behavior; and prothoracicotropic hormone (PTTH), which stimulates molting by initiating biosynthesis and release of ecdysone by the prothoracic gland and diapause hormone that arrests development in eggs.
Reproductive Nps
This family includes the ovary maturating peptide (OMP) and egg development neurosecretory hormone (EDNH), which stimulate egg development; oostatic hormone (OH), which inhibits maturation of ovaries; trypsin modulatory oostatic factor (TMOF), which regulates egg development by modulating trypsin biosynthesis in the gut; neuroparsin which affects gonad activity; PTTH, which affects egg development; and pheromone biosynthesis activating neuropeptide (PBAN), which elicits sex pheromone biosynthesis in female moths.
Homeostatic/Metabolic Nps
This group includes the adipokinetic/hypertrehalosaemic pep-tides (AKH/HT) that regulate carbohydrate and lipid metabolism, and bombyxin and other insulin-related peptides that control fat, carbohydrate, and protein metabolism. Additional members of the family are the diuretic and antidiuretic hormone peptides and the ion-transporting peptides (ITP), which are involved in osmoregula-tion, regulation of Malpighian tubule activity, and water balances.
Myotropic Nps
This family is one of the largest Np families in insects; it regulates the contractile activity of visceral and/or skeletal muscles. The family includes peptides such as proctolin, CCAP, FMRFamide-related peptides, myokinins, sulfakinins, pyrokinins, tachykinins short and long neuropeptide F (NPF), myotropins and myoinhibitory peptides (e.g., allatostatin and myosuppressin).
Chromatotropic and Circadian Nps
Members of this family include melanization and reddish coloration hormone (MRCH), pigment dispersing hormone/factor (PDH/ PDH), and corazonin, a cardioactive peptide that exhibits dark pigmentation properties.
Most of the above-mentioned insect Nps have been characterized, their amino acid sequences have been determined, and their cDNA and genes have been cloned from various insect species. The analysis of the structures and modes of action of major Np families of insects has revealed that some peptides (e.g., AKH, PBAN) belong to families that are known to occur only in arthropods. These studies also revealed that a few peptides (e.g., proctolin and CCAP) belong to “one-member” families, whereas others are members of large families (e.g., the PK/PBAN family). Moreover, for some Nps gene duplication occurred resulting in the presence of more than one Np form (isoform) in a given species (e.g., AKH, allatostatins, and myotropic and FMRF-related peptides). Many Nps are multifunctional and elicit more than one biological response in a single insect species or in several different ones; and a given biological activity may be regulated in a given insect species by more than one peptide. A detailed review of the structural, biochemical, and physiological characterization of insect Nps (based on structural and functional characteristics) has been presented by Gade and by Gade and Marco. Recently, with the availability of the genomic data, an alternative approach for Np categorization, based on Np precursor genes and their peptides has been introduced. This approach was summarized by Altstein and Nassel.
NOMENCLATURE
The nomenclature of insect Nps is usually based on two primary characteristics: the Np source, which is indicated by the first two letters of the genus name (with the first letter capitalized) and the first letter of the species name, and the first reported or the major biological function. For example, a peptide isolated from Helicoverpa zea which was first reported to have a pheromonotropic activity, would be designated Hez-PBAN. A detailed explanation of insect peptide nomenclature was presented by Raina and Gade.
CELLULAR AND MOLECULAR ASPECTS OF NP ACTIVITY
The cellular and molecular components of Np activity include biosynthesis, release, transport, activation of the target cell, and inac-tivation of the Np to terminate its action. Np gene expression is regulated by a complex series of factors that are controlled by other Nps and neurotransmitters. The Nps are synthesized as large precursor polypeptide chains (termed preprohormones) that include a signal sequence which is removed by an endopeptidase during translation. The remaining protein, the prohormone, is transported to the Golgi apparatus, where it is packed into secretory vesicles in which it is further processed proteolytically into smaller fragments by prohormone-converting enzymes. Precursors can encode multiple bioactive Nps within a single molecule, including replicates of an individual peptide or single copies of numerous peptides. During the transit through the Golgi network, the precursors may be subjected to post-translational modifications such as glycosylation, phosphorylation, sulfation, or hydroxylation. Secretory vesicles containing Nps accumulate at NSC terminals and are secreted by a regulatory secretory pathway (usually in a Ca2+-dependent manner). Unlike neurotransmitters, Nps are not recycled at the nerve terminals but are newly synthesized in the cell body. Following secretion (either to the circulatory system via neurohemal organs, or to the intracellular space of adjacent cells) the Nps reach their target organ, where they activate their target cells by binding to cell-surface proteins (termed receptors) and exciting second-messenger systems, thus eliciting a variety of cellular responses. At the end of the activation, Nps dissociate from the receptor and are rapidly inactivated by peptidases present in the plasma, in the intercellular space, or in the target cell membrane. All the above events are common to all Nps, regardless of their origin or biological function. Strand has provided a detailed summary of these processes.
NP RECEPTORS
Nps activate cellular processes via GPCRs, which are seven trans-membrane domain proteins (7TM) that sense molecules outside the cell and activate internal signal transduction pathways and, ultimately, cellular responses. Prior to the determination of insect genomes, receptors were isolated from the relevant tissue and characterized biochemically and pharmacologically. This approach has led to the characterization of a dozen different receptors from a variety of tissues in diverse insects: locust hindgut proctolin receptor; Blaberus craniifer fore- and hindgut proctolin receptor; M. sexta Malpighian tubules diuretic hormone receptor and fat body AKH receptor; Diploptera punctata brain and midgut allatostatin receptors; B. mori ovarian cell bombyxin receptor; locust oviduct FLRFamide receptor; cricket Malpighian tubule achetakinin receptor; locust and flesh fly tachykinin receptors; A. aegypti Malpighian tubule leucokinin receptor; and Heliothis peltigera pheromone gland pyrokinin/PBAN receptor. Two of the above receptors were cloned (diuretic hormone and tachyki-nin). An outburst of studies on insect Np receptor cloning occurred in the late 1990s, when databases of various gene banks, and especially that of the Berkeley Drosophila Genome Project, became available to the scientific community. In less than 4 years eight novel insect Np receptors have been cloned by means of a variety of approaches. Some receptors (the cattle tick Boophilus microplus leucokinin receptor and the D. melanogaster tachykinin and neuropeptide Y receptors) were cloned by PCR with primers based on the 7TM domain common motifs. Other approaches included screening cDNA and genomic libraries with sequences homologous to mammalian receptors (e.g., Drosophila growth hormone receptor, and allotostatin-1 and -2 receptors) or receptors of related families isolated from other insects (e.g., Drosophila and stable fly tachykinin receptor, P. americana allatostatin receptor). The field received an even greater boost with the completion in 2000 of the Drosophila genome sequence and subsequently of those of other insects. Within a few years, 48 Np receptor genes that represent the vast majority, perhaps all, of the peptide GPCRs encoded by the fly genome have been predicted and, to date, nearly 30 D. melanogaster GPCRs have been characterized with respect to their preferred peptide ligands. Peptides from other insects, too, have been identified, and recently knowledge on D. melanogaster GPCRs was used to annotate 35 Np GPCRs in the recently sequenced genome of the honey bee, A. mellifera. A detailed summary was presented by Hauser et al.
SUMMARY AND FUTURE PROSPECTS
The combination of the above novel approaches (e.g., MS, large-scale peptidomic studies, genome sequence analysis, and targeted mutagen-esis of Np genes) with the more classical immunochemical, biochemical, and cytochemical approaches, together with )n vitro studies of characterization of peptide GPCRs and analysis of their distributions in various insects has provided us with a great source of information and has significantly advanced our understanding of Np signaling. Further studies along these lines, combined with comparative experimental studies, based on information from annotated genomes of multiple insect species should be pursued in order to achieve identification and structural and functional characterization of some orphan receptors and predicted Nps, which should lead to evaluation of the functional role of Np isoforms in the organism, and enable study of the various downstream secondary messenger pathways all of which can be expected to further improve our insight into the various aspects of Np signaling. Altstein has recently summarized a novel approach to the exploitation of this avenue.
In addition to their position as prime objectives in advancing our understanding of the physiology of insects, Nps and their receptors are also targets for the development of novel insect-control strategies based on interference with their activity by means of receptor-specific agonists or antagonists. The use of Nps as a basis for insecticide design made great progress in the past decade because of the vast input of novel information on GPCRs, Np genes, and their sequences. This information provides a basis for development of insect-control agents based on rational/structural design. This field is still in its infancy and, although this strategic approach has been used to develop a few vertebrate Np agonists and antagonists, the technology has not yet been optimized. Thus, new approaches to the generation of Np agonists/antagonists and to their further conversion into insecticide prototype compounds with specific desired features need to be developed. Once these strategies are devised, it should be possible to implement them with a variety of Nps in order to tailor highly potent insect-control agents.
