Muscle is the excitable, contractile tissue of animals that is responsible for movement and behavior. Although there is great variability in structure and performance among different insect muscles, many basic features of biochemical composition, ultrastructural organization, and contractile performance are common among insect muscles and indeed are similar between muscles of insects and those of vertebrates.
MUSCLE STRUCTURE AND ULTRASTRUCTURE
Muscle Fibers and Fiber Bundles
The skeletal muscles of insects are bundles of elongate, multinu-cleate cells called muscle fibers. The fibers attach at each end to the exoskeleton. The muscles typically span joints of the exoskeleton and, when active, cause bending of the joint or stabilization of the joint against external forces. Skeletal muscles are the muscles of behavior, the muscles involved in posture and locomotion. In addition to skeletal muscles, insects contain visceral muscles that cause movement of the gut, Malpighian tubules, and parts of the reproductive system; there are also cardiac muscles that cause contraction of tissue sheets and vessels associated with the circulatory system. The visceral and cardiac muscle cells are typically small, spindle shaped, and with a single nucleus.
An individual insect contains many morphologically identifiable skeletal muscles. The large number of muscles is a consequence of the segmental organization of insects and the serial replication of parts associated with segmentation. Each of the wing-bearing segments of a cockroach contains about 50 separate muscles, an abdominal segment a somewhat smaller number. In a classic anatomical study, Lyonet, in 1762, noted that the larva of the goat moth, Cossus, contains three times the number of anatomically distinct skeletal muscles as does a human!
In most insect muscles the fibers lie parallel to one another, and when the muscle contracts, it shortens along the long axis of the fiber bundle. Such muscles are spoken of as being parallel-fibered muscles (Fig. 1, left). In some muscles, in particular peripheral leg muscles, the fibers attach obliquely at one of their ends onto an internal, cuticular extension called an apodeme (Fig. 1, right). When these muscles are activated, the muscle as a whole shortens along the axis of the apodeme, oblique to the fiber axis. The oblique insertion of fibers onto the apodeme is remindful of the oblique junction between lateral filaments and the main shaft of a feather, hence muscles with an oblique fiber arrangement are called pinnate (L. pinna = feather). The force that a muscle can generate increases with increasing cross-sectional area. The pinnate arrangement of muscles increases the effective cross-sectional area and hence the force that the muscle can produce.
Filaments and Fibrils
Muscle shortening in insects as in other animals results from sliding movement between interdigitating thick and thin filaments
![Muscle with parallel fibers (left) and one with pinnate fibers (right). The parallel-fibered muscle is the mesothoracic dorsal longitudinal flight muscle of the tettigoniid Neoconocephalus robustus. [Modified from Stokes, Josephson and Price (1975). J. Exp. Zool. 194, 379-407.] The dark structure coursing across the muscle surface is the motor nerve that innervates the muscle. The pin-nately fibered muscle is the metathoracic extensor tibia of the cricket Teleogryllus oceanicus. Abbreviations: N, motor nerve; Tr, trachea; A, apodeme. Muscle with parallel fibers (left) and one with pinnate fibers (right). The parallel-fibered muscle is the mesothoracic dorsal longitudinal flight muscle of the tettigoniid Neoconocephalus robustus. [Modified from Stokes, Josephson and Price (1975). J. Exp. Zool. 194, 379-407.] The dark structure coursing across the muscle surface is the motor nerve that innervates the muscle. The pin-nately fibered muscle is the metathoracic extensor tibia of the cricket Teleogryllus oceanicus. Abbreviations: N, motor nerve; Tr, trachea; A, apodeme.](http://lh6.ggpht.com/_X6JnoL0U4BY/S8HcJ6shuRI/AAAAAAAAYV8/NzKRxgdjMIw/tmp910_thumb_thumb.jpg?imgmax=800)
FIGURE 1 Muscle with parallel fibers (left) and one with pinnate fibers (right). The parallel-fibered muscle is the mesothoracic dorsal longitudinal flight muscle of the tettigoniid Neoconocephalus robustus. [Modified from Stokes, Josephson and Price (1975). J. Exp. Zool. 194, 379-407.] The dark structure coursing across the muscle surface is the motor nerve that innervates the muscle. The pin-nately fibered muscle is the metathoracic extensor tibia of the cricket Teleogryllus oceanicus. Abbreviations: N, motor nerve; Tr, trachea; A, apodeme.
contained within the muscle fibers. The force of contraction is a shearing force developed between these filaments. The thick filaments are made up largely of the protein myosin, the thin filaments of the protein actin. A single thick filament is composed of many individual myosin molecules and, similarly, a thin filament contains many actin molecules. Projections of the myosin molecules from the thick filaments toward the thin filaments, called cross-bridges, are the sites of interaction between the two and are the force generators for contraction.
The thick and thin filaments are grouped into longitudinal bundles called fibrils. The filaments within a fibril are grouped precisely, both longitudinally and transversely ( Figs. 2 and 3 ). The thin filaments attach to and project from both sides of transverse structures called Z disks. The Z disks occur regularly along the length of the fibril. The interval from one Z disk to the next is called a sarcomere. The thick filaments lay side by side in the middle of the sarcomere. The sarcomere lengths in fibrils of fast muscles such as flight muscles are 2-4 |im; those in leg muscles, body wall muscles, and visceral muscles tend to be longer, up to 7-10 |im. The regular longitudinal arrangement of Z disks, thin filaments, and thick filaments creates a striped pattern along the length of a fibril (Figs. 2 and 3). The most obvious components of the striped pattern are (1) the Z disks; (2) the A bands, corresponding to that part of a sarcomere containing thick filaments; and (3) the I bands, corresponding to that part of a sarcomere without thick filaments. When a muscle shortens, the thin filaments slide toward the center of the sarcomere and the I bands become shorter. Because of their transverse banding pattern, muscles in insects (and skeletal muscle in vertebrates, which have a similar organization) are described as being striated muscles. The visceral muscles of insects are similar in function to vertebrate smooth muscles and in many ways similar in physiology as well. But although vertebrate smooth muscles lack

FIGURE 2 Origin of the transverse striations in skeletal muscle. The upper electron micrograph is a longitudinal section of a somewhat stretched fiber from the mesothoracic dorsal longitudinal muscle of the tettigoniid Neoconocephalus ensiger. The scale bar represents 1 |im. Abbreviations: M, mitochondrion; I, I band; Z, Z disk; A, A band.
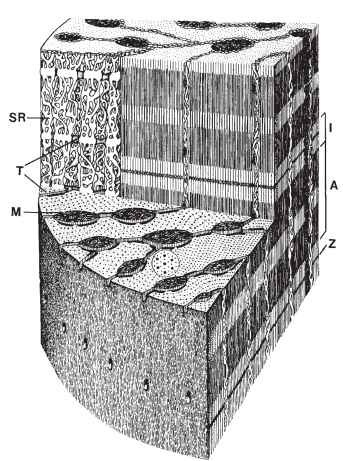
FIGURE 3 Structural organization of a fiber from an insect fast muscle. The drawing is based on electron micrographs from a tettigoniid singing muscle. The fibrils here are radial-lamellar. Abbreviations: A, A band; I, I band; M, mitochondrion; SR, sarco-plasmic reticulum; T, transverse tubule; Z, Z disk.
striations, the visceral muscles of insects, like the skeletal muscles, are striated.
The thick filaments of insect muscles, and of vertebrate striated muscles, occur in a regular, hexagonal array. In vertebrate muscles a thick filament is surrounded by six thin filaments, each of which lies at the midpoint between three adjacent thick filaments (Fig. 4), and the overall ratio of thin-to-thick filaments is 2:1. In fast muscles of insects, for example flight muscles, there are also six thin filaments surrounding each thick filament, but these occur at the midpoint between two thick filaments and the thin-to-thick ratio is 3:1. In slower insect muscles, such as body wall muscles, the usual pattern is for each thick filament to be surrounded by a circle of up to 12 thin filaments.
The fibrils of insect muscles occur in two basic patterns, cylindrical and radial-lamellar (Figs. 3 and 5). In muscles with cylindrical fibrils the bundles of filaments forming the fibrils occur as elongate cylinders that are often polygonal in cross section. In radial-lamellar fibers the fibrils are ribbon-shaped structures arranged radially about the center of the fiber.
Other Components
The cellular components of muscle fibers seen in electron micrographs fall into four functional groups. First are those structures directly involved in the generation of force and mechanical power.
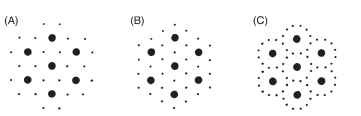
FIGURE 4 Organization of thick and thin filaments as seen in cross sections of fibers from (A) a vertebrate skeletal muscle, (B) a fast insect muscle, and (C) a slow insect muscle.
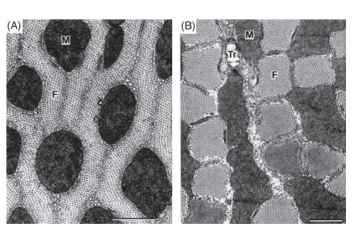
FIGURE 5 Transverse electron microscope sections through (A) a fiber with radial-)amellar fibrils (a flight muscle of the tettigoniid Euconocephalus nasutus) and (B) a fiber with columnar fibrils (from the tymbal muscle of the cicada Abricta curvicosta). The scale bars indicate 1 |im. Abbreviations: M, mitochondrion; F, fibril; Tr, intrac-ellular tracheole.
These structures are the thick and thin filaments that collectively form the fibrils. Second are those components involved in the control of contraction. The most obvious structures involved in the control of contraction are the transverse tubular system (T tubules) and the sarcoplasmic reticulum (SR). The T tubules are membrane-bound tubular structures oriented perpendicular to the fiber axis. The membrane of a T tubule is continuous with the surface membrane of the fiber, and the T tubule can be regarded as an inwardly directed extension of the surface membrane. In most insect muscles there are two tubules per sarcomere, lying in the overlap areas between thick and thin filaments (Fig. 3), but in the fibers of some muscles there is a single, centrally located T tubule per sarcomere. Within the fiber the T tubules make specialized junctions with the SR, which is an internally closed, membrane-bound compartment within the fiber. The function of the T tubules and SR is considered further below. Other elements involved in the control of contraction are the surface membrane of the muscle fibers and membrane specializations at the sites at which nerve processes contact muscle fibers. Third are the structural elements of the metabolic power supply. These are the mitochondria, which provide ATP, and glycogen granules. ATP is the immediate energy source for contraction; glycogen is a stored fuel for cellular metabolism. It would be appropriate to include among the elements involved in metabolic power the trache-oles, the terminal portions of the gas-exchange system that ramify throughout muscle fibers, even though topologically tracheoles are external to and not really part of the muscle fibers. Fourth are the structures involved in long-term maintenance of muscle, specifically the many nuclei of the fibers.
The relative abundance of different cellular components in muscle is tightly correlated with the functional capacity of the muscle fibers. SR and T tubules are particularly abundant in muscles that can produce brief contractions, that is, in muscles in which the contractile apparatus can be rapidly activated and inactivated. Muscles capable of sustained activity at high power output are particularly well supplied with mitochondria and tracheolar endings. Mitochondria make up 30-40% of the muscle volume in wing muscle of active fliers and in sound-producing muscles that are active continuously and at high frequency. Such muscles are often pink, because of the cytochromes in the abundant mitochondria. It should be noted that hypertrophy of mitochondria, and of T tubules and SR, is at the expense of myofibrillar volume, so fast and fatigue-resistant muscles are likely to be relatively weak.
Muscle Attachments
Skeletal muscles attach to the cuticle of the exoskeleton through specialized epidermal cells. The muscle fibers are joined to these cells by specialized junctions called desmosomes. The terminal sarcomeres of the fibrils lack a final Z disk; instead, the thin filaments are attached to the muscle portion of the terminal desmo-some through a band of what have been called junctional filaments. Visceral muscles are frequently joined to one another by desmo-somes, and cardiac muscle fibers are joined by structures resembling the intercalary disks of vertebrate cardiac muscle.
INNERVATION AND ACTIVATION
There is an electrical potential across the surface membrane of a living, resting muscle fiber; the interior of the fiber is typically 3070 mV electrically negative with respect to the extracellular solution. Nerve cells in the central nervous system send out long processes (motor axons) to the muscle fibers where they make specialized contacts termed synapses. A motor axon makes many synaptic contacts along the length of each muscle fiber that it innervates (multitermi-nal innervation), and a single muscle fiber may receive inputs from more than one motor axon (polyneuronal innervation). Impulses initiated in the central nervous system travel along the motor axons and cause the release of specific chemical signals (transmitters) from the motor axon terminals at the synapses. The transmitter released from the terminals of most motor axons leads to a reduction (depolarization) in the transmembrane potential of the muscle fiber in the vicinity of the nerve terminal. Muscle fiber depolarization initiates contraction of the fiber. Motor axons that depolarize muscle fibers and cause muscle contraction are called excitatory axons. Some axons, termed inhibitory axons, release transmitters that stabilize the transmembrane potential of the muscle fiber or even make it greater, thus antagonizing excitatory inputs. In addition to excitatory and inhibitory neural inputs, many muscles receive inputs from modu-latory motor neurons, activity that releases chemicals that modify muscle performance, for example, increasing muscle force and work output or speeding relaxation.
Insects, like other arthropods, manage their muscles using relatively few motor neurons. Some major muscles, for example tymbal muscles of cicadas, are innervated by a single motor neuron. Many muscles receive two to four motor neurons. The largest number of motor neurons yet described for an insect muscle is 16, to the flexor muscle in the leg of a locust.
The processes linking membrane depolarization and contractile activation have been little studied in insect muscles, but the ultrastructure, biochemistry, and contractile performance of insect muscle are so similar to those of the far better studied frog, cat, and rodent muscles that one can predict with confidence that the basic principles worked out for vertebrate muscles apply to insects as well. The expected scheme is as follows. Membrane depolarization spreads inwardly into the fiber along the T tubules. Depolarization of the T tubules, which are coupled to the SR through specialized junctions, leads to release of calcium from the SR. Released calcium reversibly binds to regulatory sites in the fibrils, turning on the contractile machinery. Relaxation occurs as the SR takes up the released calcium and reduces the calcium concentration in the cytoplasm below that needed for contractile activity.
MUSCLE MECHANICS
Muscle Force and Muscle Length
The muscle contraction initiated by a single stimulus, or by a single impulse in an innervating motor neuron, is termed a twitch; that evoked by repetitive input at a frequency high enough to maintain full activation of the muscle is termed a tetanus. A response in which a stimulated muscle develops force while held at constant length is called an isometric contraction. The isometric force generated by a muscle stimulated to contract in a tetanus is maximal at about the normal muscle length in the insect body and declines at longer and shorter lengths. The decline in force with increasing muscle length beyond the optimum is thought to be caused by a reduction in the overlap between the thick and the thin filaments and therefore in the number of myosin cross-bridges that can interact with the actin filaments. The decrease in force at short muscle lengths is probably a consequence of the thick filaments running into and being impeded by the Z disks, of collision of thin filaments in the middle of the sarcomere, and, at still shorter lengths, of overlap of thin filaments with portions of thick filaments of inappropriate polarity on the far side of the center of the sarcomere.
Some muscles in insects and elsewhere can shorten to a small fraction of their resting length, a response termed supercontrac-tion. The capacity for supercontraction appears to involve modifications in the structure of the Z disk such that there is not a collision between the Z disks and the thick filaments at short muscle lengths. In the supercontracting muscles that have been examined, the Z disk becomes perforate at short muscle lengths and the thick filaments slide through the spaces in the disks.
The posterior, intersegmental, abdominal muscles of female locusts are of particular interest for the wide range of lengths over which they can operate. During oviposition, appendages on the end of the abdomen dig and pull the posterior abdomen down into a relatively deep hole. The intersegmental muscles become stretched to about nine times their resting length. During this stretch, called superextension, the Z disks become broken up into discontinuous, nonaligned elements to which the thin filaments are attached. Muscle contractility is not lost, and contraction of intersegmental muscles returns the abdomen to its normal length following oviposi-tion. The latter part of the recovery may be supercontraction, for in the resting state the posterior intersegmental muscles are normally supercontracted, with thick filaments protruding through gaps in the Z disks.
Force, Shortening Velocity, and Power
There is an inverse relationship between the force on a muscle and the velocity with which it can shorten, a relationship conveniently expressed in a force-velocity plot (Fig. 6). To facilitate comparison of muscles of differing size, force in a force-velocity plot is usually expressed as stress (force per unit cross-sectional area) and shortening velocity as strain rate (shortening velocity per unit muscle length). Two points on a force-velocity plot are frequently used to characterize a muscle’s contractile properties: the maximum isometric stress of the muscle (Fmax, the intercept of the curve with the 0 velocity axis) and the maximum shortening velocity (Vmax, the intercept of the curve with the 0 force axis). Values for the maximum force in insect muscles are generally 5-35 N cm-2 (N, Newton; 1 N is approximately the downward force exerted by a mass of 100 g in the gravitational field at the earth’s surface). The few available measurements of the maximum shortening velocity for insect muscles, all from fast muscles, are on the order of 3-15 lengths s.
The product of force and shortening velocity has dimensions of force X distance per time (work per time) and is the rate of doing work, that is, the mechanical power output. The product of stress (force per area) and strain rate (shortening distance per second per unit muscle length) is the mechanical power per unit volume of muscle, which is readily convertible to power output per unit muscle mass. Thus each point on a force-velocity plot (or a plot of stress against strain rate) represents a power output. The power predicted from a force-velocity curve is the instantaneous power output. The peak instantaneous power is substantially greater than the sustainable power from a muscle, for during maintained activity a muscle goes through repeated contraction-relaxation cycles and therefore shortens and produces power for only part of the total time. For fast muscle, including fast insect muscles, the peak power output is 100-500Wkg-1(lW= ljs-1 = INms-1).
Muscles in insects may be divided into synchronous muscles and asynchronous muscles on the basis of the relationship between the patterns of neural activation and of contraction (see below). The
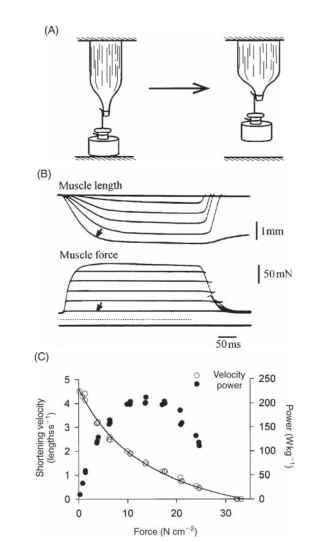
FIGURE 6 Relationships between muscle force, shortening velocity, and power output. (A) Elements of a method used to determine the relationship between force and shortening velocity. The muscle is attached to a load that is supported from below. When stimulated the muscle develops force without shortening until the force equals that of the load, following which the muscle shortens under constant load. (B) Results from an experiment examining force-velocity relations using a tettigoniid wing muscle. The lowest trace indicates the times at which the muscle was stimulated. The force trace marked by an arrow is the contraction with the smallest load of the series; the corresponding shortening trace, which has the shortest latency and the highest initial velocity, is similarly marked. (C) Force-velocity plot and a corresponding plot of power output for a wing muscle of the locust Schistocerca americana, 30°C.
sustainable power available during repetitive, cyclic contraction has been determined for several synchronous insect muscles using the work loop approach, in which the muscle is subjected to length changes simulating those during normal activity and stimulated pha-sically during the length cycles. A plot of muscle force against muscle length for a full cycle produces a loop, the area of which is the net work output of the muscle for that cycle. The product of work per cycle and cycle frequency is the power output. The mechanical power available from synchronous flight muscles of several locusts and katydids and of a moth measured in this way ranges from 50 to 120 Wkg-1 at normal operating temperature.
Asynchronous and Synchronous Muscles
Most insect muscles are like vertebrate skeletal muscles in that each contraction is initiated by depolarization of muscle fibers, and there is a 1:1 relationship between muscle electrical activity and muscle contraction. Such muscles may be termed synchronous muscles, reflecting the congruence between electrical and mechanical activities. The major flight muscles in several insect groups are different in that there is no synchrony between electrical and mechanical events. These muscles are known as asynchronous muscles. Neural input and fiber depolarization are needed to activate an asynchronous muscle, but when it is activated an asynchronous muscle can contract in an oscillatory manner if it is attached to a mechanically resonant load. The resonant loads for the flight muscles are the wings, which may be regarded as small, somewhat dampened tuning forks. The frequency of the oscillatory contraction is the mechanically resonant frequency of the load, which is greater than the neural input frequency required to keep the muscle fully activated. The contraction frequency of asynchronous wing muscles during flight is typically 3-10 times higher than the neural input frequency in each of the motor neurons activating the muscle. The main singing muscles in some but not all cicadas are asynchronous muscles; the resonant load here is the cuticular tymbal to which the muscle is attached and whose inward movement produces the sound pulses.
The features of asynchronous muscle that allow oscillatory contraction are stretch activation and shortening deactivation. When allowed to shorten rapidly an asynchronous muscle becomes deactivated, and while deactivated it can be stretched out to its original length, developing less force than it did while shortening. Stretching the muscle, in turn, reactivates it. Because of shortening deactiva-tion, less work is required to restretch an asynchronous muscle than is produced by the muscle during shortening, and there is net work output when the muscle undergoes a shortening-lengthening cycle. It is this net work that is available to drive the wings and power flight.
Asynchronous muscles occur in several of the most successful insect groups. They power flight in beetles, flies, bees, and wasps and many of the true bugs. The distribution of asynchronous muscles among insect taxa suggests that this mode of muscle control has evolved independently as many as 7-10 times. It is likely that asynchronous muscle has been favored by evolution because it is more powerful and more efficient than is synchronous muscle for operation at the high frequencies characteristic of insect flight. It is more powerful, in part, because asynchronous control does not demand rapidity in the rate at which muscle is turned on and off by neural input. High-frequency contraction is achieved without hypertrophy of the SR, leaving more room in muscle fibers for fibrils, which are the power-producing component. It is more efficient because a relatively low-frequency neural input is needed to maintain full activation, which reduces the amount of calcium that is released and rebound during activity and the associated metabolic costs of calcium cycling.
Are Insect Muscles Unusual as Motors?
In the minds of many people, insects are extraordinary athletes. One sometimes hears it said that if a person were as strong as an insect, he or she could carry enormous weights or leap over tall buildings. Such assertions are largely based on incorrect application of principles of scaling. Consider, for example, jumping ability. A 1-g locust can develop enough power to lift its 1 g of mass to a height of about 1 m. A 70-kg person can develop enough power in a jump to lift his or her 70 kg to a height of 1 m. The work done is 1 g m for the locust, 70 kg m for the person, and the power required per mass of animal is the same.
The most often studied and certainly the most completely analyzed muscle for any animal is the frog sartorius muscle. The most complete body of information on contractile properties for an insect muscle is probably for the wing muscles of locusts, both Schistocerca gregaria and S. americana. The frog sartorius muscle is not the strongest or the fastest vertebrate muscle known, but it is a good representative of a fast vertebrate muscle. Similarly locust flight muscles are neither the strongest nor the fastest insect muscles, but they are reasonable representatives of fast insect muscles. Some of the contractile properties of frog and locust muscle are tabulated in Table I. The vertebrate muscle and the insect muscle are surprisingly similar in many of their contractile properties. The muscles of insects share the same capacities and are subject to the same limitations as are muscles elsewhere throughout the animal kingdom.
Table I |
||
Contractile Properties of a Locust Flight Muscle (Metathoracic |
||
| Second Tergocoxal Muscle of S. americana) and the Frog | ||
| Sartorius Muscle | ||
| Locust | Frog | |
| Twitch time course (ms) | ||
| Rise time | 20 | 21 |
| Onset to 50% relaxation | 39 | 52 |
| Tension (Ncm-2) | ||
| Twitch | 17 | 5 |
| Tetanic | 30 | 25 |
| Maximum shortening velocity (length s-1) | 4.1 | 6.4 |
| Power (Wkg-1) | ||
| Peak instantaneous | 150 | 250 |
| Cyclic, sustained | 48 | 50 |
| Note. Values were collected at 25°C or adjusted to the expected value at 25°C | ||
| from measurements made at 20°C or 30°C using an | assumed Q10 of 2. Locust | |
| data are from Malamud, Mizisin, and Josephson (1988). J. Comp. Physiol. A | ||
| 162, 827-835 and Malamud, unpublished; frog data | are from Renaud and | |
| Stevens (1981). Am. J. Physiol. 240, R301-R309, Rome (1983). Physiol. Zool. | ||
| 56, 33-40, and Stevens (1988). J. Muscle Res. Cell Motil. 9, 329-333. | ||
